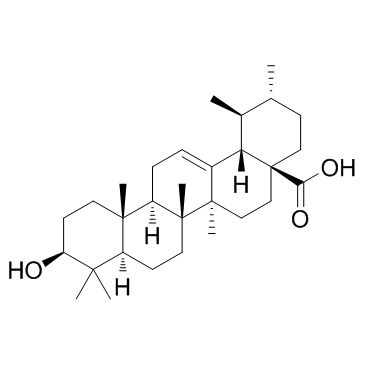
Uvaol
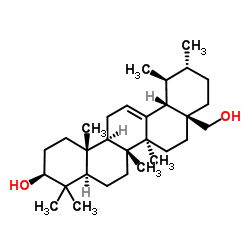
Uvaol structure
|
Common Name | Uvaol | ||
|---|---|---|---|---|
| CAS Number | 545-46-0 | Molecular Weight | 442.717 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 523.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H50O2 | Melting Point | 223-225ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 211.6±24.7 °C | |
Use of UvaolUvaol, a triterpene present in olives and virgin olive oil, possesses anti-inflammatory properties and antioxidant effects. Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice[1]. |
| Name | uvaol |
|---|---|
| Synonym | More Synonyms |
| Description | Uvaol, a triterpene present in olives and virgin olive oil, possesses anti-inflammatory properties and antioxidant effects. Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 523.7±50.0 °C at 760 mmHg |
| Melting Point | 223-225ºC(lit.) |
| Molecular Formula | C30H50O2 |
| Molecular Weight | 442.717 |
| Flash Point | 211.6±24.7 °C |
| Exact Mass | 442.381073 |
| PSA | 40.46000 |
| LogP | 9.13 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.550 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Journal of the American Pharmaceutical Association (1912-1977), , vol. 39, p. 475 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Zakaria, Muhamad Bin; Jeffreys, J. A. D.; Waterman, Peter G.; Zhong, Shou-Ming Phytochemistry (Elsevier), 1984 , vol. 23, # 7 p. 1481 - 1484 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Collins, Dwight O.; Ruddock, Peter L. D.; Grasse, Jessica Chiverton de; Reynolds, William F.; Reese, Paul B. Phytochemistry (Elsevier), 2002 , vol. 59, # 5 p. 479 - 488 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Majumder, P. L.; Bagchi, A. Tetrahedron, 1983 , vol. 39, # 4 p. 649 - 656 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 31, # 5 p. 1567 - 1571 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 31, # 5 p. 1567 - 1571 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 31, # 5 p. 1567 - 1571 |
|
~% 
Uvaol CAS#:545-46-0 |
| Literature: Journal of the American Pharmaceutical Association (1912-1977), , vol. 34, p. 39,40 |
|
Changes of the wax contents in mixtures of olive oils as determined by gas chromatography with a flame ionization detector.
J. AOAC Int. 95(6) , 1720-4, (2012) Mixing of refined olive-pomace oil with virgin olive oil is a fraud that has been tried often. Normally, the tests that detected the fraud were determinations of wax esters, erythrodiol+uvaol, and sti... |
|
|
Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells.
Cytokine 36(5-6) , 211-7, (2006) Olive pomace oil, also known as "orujo" olive oil, is a blend of refined-pomace oil and virgin olive oil, fit for human consumption. Maslinic acid, oleanolic acid, erythrodiol, and uvaol are pentacycl... |
|
|
Study of skin anti-ageing and anti-inflammatory effects of dihydroquercetin, natural triterpenoids, and their synthetic derivatives.
Bioorg. Khim. 38(3) , 374-81, (2012) Accessible triterpenoids of ursane and lupane series, the flavonoid dihydroquercetin and their synthetic derivatives with polar substituentss were tested in vitro for inhibition of collagenase 1 (MMP-... |
| urs-12-ene-3,28-diol |
| Urs-12-ene-3,28-diol, (3β)- |
| (3S,4aR,6aR,6bS,8aS,11R,12S,12aS,14aR,14bR)-8a-(Hydroxymethyl)-4,4,6a,6b,11,12,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picenol |
| urs-12-ene-3beta,28-diol |
| 12-URSEN-3BETA,28 DIOL |
| 3B,28-DIHYDROXY-URSA-12-EN |
| (3β)-Urs-12-ene-3,28-diol |
| 12-URSEN-3B,28-DIOL |
| Uvaol,Urs-12-ene-3,28-diol |
| (3S,4aR,6aR,6bS,8aS,11R,12S,12aS,14aR,14bR)-8a-(Hydroxyméthyl)-4,4,6a,6b,11,12,14b-heptaméthyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picénol |
| Urs-12-ene-3β,28-diol |
| Urs-12-ene-3b,28-diol |
| Uvaol |
| MFCD00009620 |