Vanillyl mandelic acid
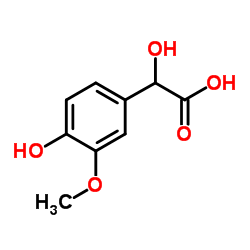
Vanillyl mandelic acid structure
|
Common Name | Vanillyl mandelic acid | ||
|---|---|---|---|---|
| CAS Number | 55-10-7 | Molecular Weight | 198.173 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 421.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C9H10O5 | Melting Point | 132-134 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 173.7±22.2 °C | |
Use of Vanillyl mandelic acidVanillylmandelic acid is the endproduct of epinephrine and norepinephrine metabolism. Vanillylmandelic acid can be used as an indication of the disorder in neurotransmitter metabolism as well. Vanillylmandelic acid has antioxidant activity towards DPPH radical with an IC50 value of 33 μM[1]. |
| Name | vanillylmandelic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Vanillylmandelic acid is the endproduct of epinephrine and norepinephrine metabolism. Vanillylmandelic acid can be used as an indication of the disorder in neurotransmitter metabolism as well. Vanillylmandelic acid has antioxidant activity towards DPPH radical with an IC50 value of 33 μM[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Vanillylmandelic acid has antioxidant activity, the IC50 value is determined as the amount of VMA required to reduce 50% of the starting concentration of free radical is 33*10-6 M in the UV-Vis decolourisation DPPH assay[1]. |
| In Vivo | Vanillylmandelic acid (intra-arterial injection over 1 min; 1, 10 and 100 mg/kg; the 60 min-observation period) produces a significant difference between vanillylmandelic acid groups and controls. Vanillylmandelic acid decreases the heart rate by 17.5%, 17.9% and 18.9% after 1, 10 and 100 mg/kg, respectively. Mean blood pressure is decreased by 13.5% in control animals as compared to 37%, 23% and 26% after 1, 10 and 100 mg/kg, respectively, in wistar rats[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 421.3±45.0 °C at 760 mmHg |
| Melting Point | 132-134 °C (dec.)(lit.) |
| Molecular Formula | C9H10O5 |
| Molecular Weight | 198.173 |
| Flash Point | 173.7±22.2 °C |
| Exact Mass | 198.052826 |
| PSA | 86.99000 |
| LogP | -0.11 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.606 |
| InChIKey | CGQCWMIAEPEHNQ-UHFFFAOYSA-N |
| SMILES | COc1cc(C(O)C(=O)O)ccc1O |
| Storage condition | 2-8°C |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36-37/39 |
| RIDADR | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| RTECS | CY9033750 |
| Packaging Group | III |
| Hazard Class | 3 |
| HS Code | 29036990 |
|
~87% 
Vanillyl mandel... CAS#:55-10-7 |
| Literature: Niu, Dong-Fang; Li, Hui-Cheng; Zhang, Xin-Sheng Tetrahedron, 2013 , vol. 69, # 38 p. 8174 - 8177 |
|
~66% 
Vanillyl mandel... CAS#:55-10-7 |
| Literature: Yang, Ben-Yong; Yang, De-Hong Journal of Chemical Research, 2011 , vol. 35, # 8 p. 484 - 485 |
|
~% 
Vanillyl mandel... CAS#:55-10-7 |
| Literature: Gardner; Hibbert Journal of the American Chemical Society, 1944 , vol. 66, p. 607,609 |
|
~64% 
Vanillyl mandel... CAS#:55-10-7 |
| Literature: Varala, Ravi; Kotra, Vijay; Alam, M. Mujahid; Kumar, N. Ramesh; Ganapaty; Adapa, Srinivas R. Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2008 , vol. 47, # 8 p. 1243 - 1248 |
| Precursor 6 | |
|---|---|
| DownStream 5 | |
| HS Code | 29036990 |
|---|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Lipophilicity of amine neurotransmitter precursors, metabolites and related drugs estimated on various TLC plates.
J. Chromatogr. Sci. 52(9) , 1095-103, (2014) The retention behavior for a series of amine neurotransmitters, their precursors, metabolites and structurally related drugs has been investigated in reversed-phase thin-layer chromatography using RP-... |
|
|
A reliable and simple method for the assay of neuroendocrine tumor markers in human urine by solid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry.
Anal. Chim. Acta 759 , 66-73, (2013) Homovanillic acid (HVA), vanylmandelic acid (VMA), and 5-hydroxyindoleacetic acid (5-HIAA) are the metabolites of some catecholamines such as epinephrine, nor-epinephrine, dopamine and serotonin and t... |
| dl-α,4-dihydroxy-3-methoxyphenylacetic acid |
| Hydroxy(4-hydroxy-3-methoxyphenyl)acetic acid |
| Vanilmandelic Acid |
| 4-Hydroxy-3-methoxy-DL-mandelic acid |
| DL-VANILLOMANDELIC ACID |
| Vanilinmandelic Acid |
| DL-Vanillylmandelic acid |
| 4-Hydroxy-3-methoxyphenyl(hydroxy)acetic acid |
| QVYQR DQ CO1 |
| (4-Hydroxy-3-methoxyphenyl)glycolic acid |
| UNII:0DQI268449 |
| Vanillomandelic Acid |
| α,4-Dihydroxy-3-methoxybenzeneacetic acid |
| EINECS 200-224-0 |
| Vanillinemandelic acid |
| 4-hydroxy-3-methoxy-phenylglycolic acid |
| 3-methoxy-p-hydroxymandelic acid |
| VMA |
| VANILLYLMANDELIC ACID |
| Benzeneacetic acid, α,4-dihydroxy-3-methoxy- |
| 4-Hydroxy-3-methoxymandelic acid |
| 3-methoxy-4-hydroxymandelic acid |
| 4-Hydroxy 3-methoxymandelic acid |
| HYDROXYMETHOXYMANDELICACID |
| MFCD00004235 |
| Vanillinmandelic acid |



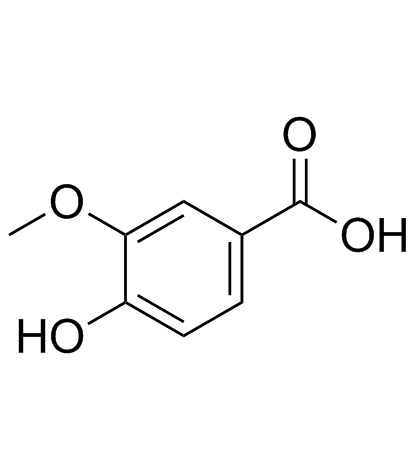 CAS#:121-34-6
CAS#:121-34-6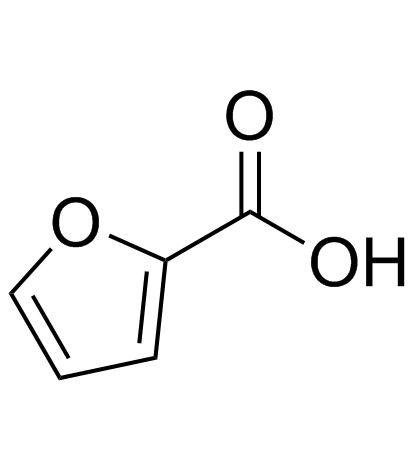 CAS#:88-14-2
CAS#:88-14-2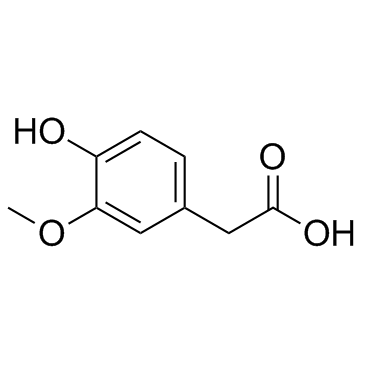 CAS#:306-08-1
CAS#:306-08-1
