4-Acetamidobenzoic acid

4-Acetamidobenzoic acid structure
|
Common Name | 4-Acetamidobenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 556-08-1 | Molecular Weight | 179.17 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 439.6±28.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO3 | Melting Point | 259-262 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 219.7±24.0 °C | |
Use of 4-Acetamidobenzoic acidAcedoben is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-acetamidobenzoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Acedoben is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 439.6±28.0 °C at 760 mmHg |
| Melting Point | 259-262 °C (dec.)(lit.) |
| Molecular Formula | C9H9NO3 |
| Molecular Weight | 179.17 |
| Flash Point | 219.7±24.0 °C |
| Exact Mass | 179.058243 |
| PSA | 66.40000 |
| LogP | 1.31 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.620 |
| InChIKey | QCXJEYYXVJIFCE-UHFFFAOYSA-N |
| SMILES | CC(=O)Nc1ccc(C(=O)O)cc1 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Comparison of xenobiotic metabolizing enzyme activities in ex vivo human skin and reconstructed human skin models from SkinEthic.
Arch. Toxicol. 88(9) , 1681-1694, (2014) Skin function is not limited to a physical barrier. According to its total surface area, it is also considered as an extra-hepatic metabolizing organ. In vitro engineered human skins have been develop... |
|
|
Regioselective carboxylation of catechol by 3,4-dihydroxybenzoate decarboxylase of Enterobacter cloacae P.
Biotechnol. Lett. 32(5) , 701-5, (2010) 3,4-Dihydroxybenzoate decarboxylase in Enterobacter cloacae P241 was induced by adding 3,4-dihydroxybenzoic acid, 3-hydroxybenzoic acid, 3,4,5-trihydroxybenzoic acid or 4-acetamidobenzoic acid to the ... |
|
|
Effects of butylated hydroxyanisole and butylated hydroxytoluene on DNA adduct formation and arylamine N-acetyltransferase activity in human bladder tumour cells.
J. Appl. Toxicol. 22(1) , 37-44, (2002) In this study, butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) were used to determine the inhibition of arylamine N-acetyltransferase (NAT) activity and DNA adduct formation in human... |
| 4-(acetylamino)benzoic acid |
| acedobene |
| Benzoic acid, 4-(acetylamino)- |
| EINECS 209-114-7 |
| p-Acetylamino benzoic acid |
| p-acetylaminobenzoic acid |
| acedobenum |
| Acedoben |
| p-Acetylamino-benzoic acid |
| N-ACETYL-PABA |
| 4-acetaminobenzoic acid |
| PAABA |
| p-Carboxyacetanilide |
| 4-acetamidobenzoic_acid |
| para-acetamidobenzoic acid |
| LR-64 |
| p-Acetaminobenzoic acid |
| ACETYLATED PABA |
| RARECHEM AL BO 0055 |
| 4-Acetylaminobenzoic acid |
| 4-Acetamidobenzoic acid |
| PAAB |
| N-acetyl-4-aminobenzoic acid |
| 4-(acetylamino)benzenecarboxylic acid |
| Acedoben [INN] |
| MFCD00002534 |
| p-acetamidobenzoic |
 CAS#:150-13-0
CAS#:150-13-0 CAS#:64-19-7
CAS#:64-19-7 CAS#:108-24-7
CAS#:108-24-7 CAS#:103-89-9
CAS#:103-89-9 CAS#:122-85-0
CAS#:122-85-0 CAS#:532-32-1
CAS#:532-32-1 CAS#:141-78-6
CAS#:141-78-6 CAS#:75-36-5
CAS#:75-36-5 CAS#:507-09-5
CAS#:507-09-5 CAS#:10602-00-3
CAS#:10602-00-3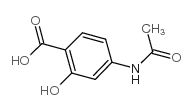 CAS#:50-86-2
CAS#:50-86-2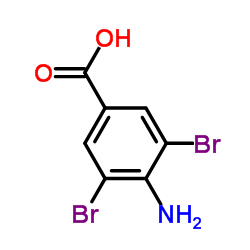 CAS#:4123-72-2
CAS#:4123-72-2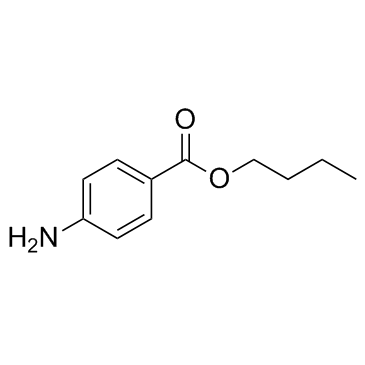 CAS#:94-25-7
CAS#:94-25-7 CAS#:190071-24-0
CAS#:190071-24-0![[(4-Acetylaminobenzoyl)oxy]tributylstannane structure](https://image.chemsrc.com/caspic/033/2857-03-6.png) CAS#:2857-03-6
CAS#:2857-03-6 CAS#:56961-25-2
CAS#:56961-25-2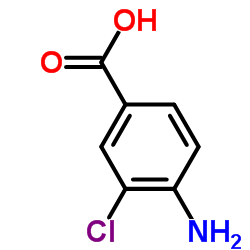 CAS#:2486-71-7
CAS#:2486-71-7 CAS#:709-19-3
CAS#:709-19-3 CAS#:153450-08-9
CAS#:153450-08-9 CAS#:94-09-7
CAS#:94-09-7
