DOXYLAMINE SUCCINATE
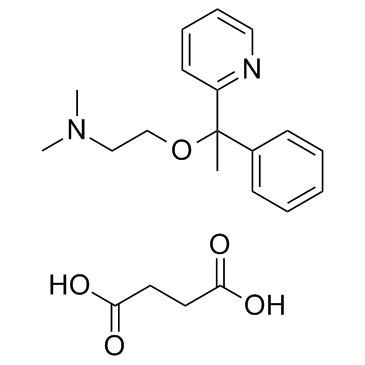
DOXYLAMINE SUCCINATE structure
|
Common Name | DOXYLAMINE SUCCINATE | ||
|---|---|---|---|---|
| CAS Number | 562-10-7 | Molecular Weight | 388.457 | |
| Density | 1.043g/cm3 | Boiling Point | 364.9ºC at 760 mmHg | |
| Molecular Formula | C21H28N2O5 | Melting Point | 103 - 108ºC | |
| MSDS | Chinese USA | Flash Point | 174.5ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of DOXYLAMINE SUCCINATEDoxylamine is a first generation antihistamine; can be used by itself as a short-term sedative and in combination with other drugs to provide night-time allergy and cold relief. |
| Name | Doxylamine succinate salt |
|---|---|
| Synonym | More Synonyms |
| Description | Doxylamine is a first generation antihistamine; can be used by itself as a short-term sedative and in combination with other drugs to provide night-time allergy and cold relief. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.043g/cm3 |
|---|---|
| Boiling Point | 364.9ºC at 760 mmHg |
| Melting Point | 103 - 108ºC |
| Molecular Formula | C21H28N2O5 |
| Molecular Weight | 388.457 |
| Flash Point | 174.5ºC |
| Exact Mass | 388.199829 |
| PSA | 99.96000 |
| LogP | 2.85910 |
| InChIKey | KBAUFVUYFNWQFM-UHFFFAOYSA-N |
| SMILES | CN(C)CCOC(C)(c1ccccc1)c1ccccn1.O=C(O)CCC(=O)O |
| Storage condition | Refrigerator |
| Stability | Stable, but may be light sensitive. Incompatible with strong oxidizing agents, acids, bases. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | US9275000 |
|
Use of In Vitro Morphogenesis of Mouse Embryoid Bodies to Assess Developmental Toxicity of Therapeutic Drugs Contraindicated in Pregnancy.
Toxicol. Sci. 149 , 15-30, (2016) In utero exposure to certain chemicals can impair embryo development, causing embryonic death, growth retardation, or severe birth defects. Establishment of effective in vitro tests is crucial for ide... |
|
|
[Nontraumatic rhabdomyolysis due to oral poisoning by doxylamine succinate].
Med. Clin. (Barc.) 108(9) , 356, (1997)
|
|
|
Safety assessment of OTC drugs: doxylamine succinate.
Arch. Toxicol. Suppl. 17 , 326-40, (1995)
|
| N,N-Dimethyl-2-[1-phenyl-1-(pyridin-2-yl)ethoxy]ethanamine succinate (1:1) |
| UNII:V9BI9B5YI2 |
| MFCD00056168 |
| butanedioic acid,N,N-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine |
| N,N-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine butanedioate |
| N,N-Dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine succinate (1:1) |
| N,N-dimethyl-2-[1-phenyl-1-(pyridin-2-yl)ethoxy]ethanamine butanedioate (1:1) |
| Succinic acid - N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine (1:1) |
| Butanedioic Acid compd. with N,N-Dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine (1:1) |
| Ethanamine, N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-, butanedioate (1:1) |
| EINECS 209-228-7 |
| Doxylamine succinate |
| Doxylamine (succinate) |