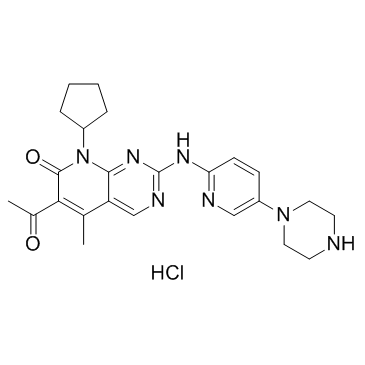
palbociclib
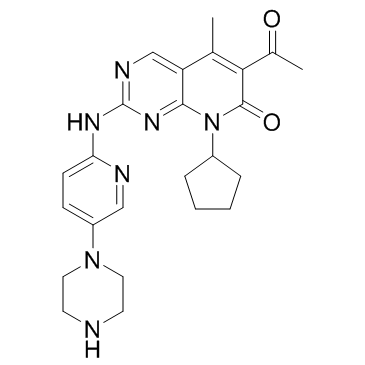
palbociclib structure
|
Common Name | palbociclib | ||
|---|---|---|---|---|
| CAS Number | 571190-30-2 | Molecular Weight | 447.533 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 711.5±70.0 °C at 760 mmHg | |
| Molecular Formula | C24H29N7O2 | Melting Point | 200ºC | |
| MSDS | N/A | Flash Point | 384.1±35.7 °C | |
Use of palbociclibPalbociclib (PD 0332991) is a selective CDK4 and CDK6 inhibitor with IC50s of 11 and 16 nM, respectively. Palbociclib is a drug for the treatment of ER-positive and HER2-negative breast cancer. |
| Name | palbociclib |
|---|---|
| Synonym | More Synonyms |
| Description | Palbociclib (PD 0332991) is a selective CDK4 and CDK6 inhibitor with IC50s of 11 and 16 nM, respectively. Palbociclib is a drug for the treatment of ER-positive and HER2-negative breast cancer. |
|---|---|
| Related Catalog | |
| Target |
Cdk4/cyclin D3:9 nM (IC50) Cdk4/cyclin D1:11 nM (IC50) Cdk6/cyclin D2:16 nM (IC50) DYRK1A:2000 nM (IC50) MAPK:8000 nM (IC50) |
| In Vitro | The IC50 of Palbociclib (PD 0332991) for reduction of retinoblastoma (Rb) phosphorylation at Ser780 in MDA-MB-435 breast carcinoma cells is 66 nM. Palbociclib is equally effective at reducing Rb phosphorylation at Ser795 in this tumor with an IC50 of 63 nM, and similar effects on both Ser780 and Ser795 phosphorylation are obtained in the Colo-205 colon carcinoma[1]. The MP-MRT-AN (AN), KP-MRT-RY (RY), G401, and KP-MRT-NS (NS) cell lines are effectively inhibited by Palbociclib (PD) in a concentration-dependent manner in a WST-8 assay. The IC50s are 0.01 µM, 0.01 µM, 0.06 µM, and 0.6 µM, respectively. In contrast, the KP-MRT-YM (YM) cell line is resistant to Palbociclib (IC50>10 µM). The flow cytometry results show that Palbociclib at concentrations between 0 to 1.0 µM induces G1 arrest in the AN, RY, G401 and NS cell lines in a concentration-dependent manner, but has no effect on YM cells. The BrdU incorporation results are consistent with the WST-8 and flow cytometry results: PD reduces BrdU incorporation (indicating G1 arrest) in the AN, RY, G401 and NS cell lines, but not in the YM cell line. Palbociclib, even at a concentration of 0.05 µM, significantly reduces BrdU incorporation in the AN, RY, and G401 cell lines (p<0.05)[2]. |
| In Vivo | Palbociclib (PD 0332991) exhibits significant antitumor efficacy against multiple human tumor xenograft models. In mice bearing Colo-205 colon carcinoma xenografts (p16 deleted), daily p.o. dosing for 14 days with Palbociclib (150 or 75 mg/kg) produces rapid tumor regressions and a corresponding tumor growth delay of ~50 days with >1 log of tumor cell kill at the highest dose tested. At 37.5 mg/kg, the tumor slowly regressed during treatment. Even at doses as low as 12.5 mg/kg, a 13-day growth delay is obtained indicating a 90% inhibition of tumor growth rate. Likewise, robust antitumor activity is seen in the MDA-MB-435 breast carcinoma (p16 deleted) where complete tumor stasis is apparent at 150 mg/kg and some cell kill is evident at the highest dose[1]. |
| Kinase Assay | CDK assays are performed in 96-well filter plates. All CDK-cyclin kinase complexes are expressed in insect cells through baculovirus infection and purified. The substrate for the assays is a fragment (amino acids 792-928) of pRb fused to GST (GST·RB-Cterm). The total volume in each well is 0.1 mL containing a final concentration of 20 mM Tris-HCl, pH 7.4, 50 mM NaCl, 1 mM dithiothreitol, 10 mM MgCl2, 25 μM ATP (for CDK4-cyclin D1, CDK6-cyclin D2, and CDK6-cyclin D3) or 12 μM ATP (for CDK2-cyclin E, CDK2-cyclin A, and CDC2-cyclin B) containing 0.25 μCi of [γ-32P]ATP, 20 ng of enzyme, 1 μg of GST·RB-Cterm, and Palbociclib (0.001-0.1μM). All components except the [γ-32P]ATP are added to the wells, and the plate is placed on a plate mixer for 2 min. The reaction is started by adding the [γ-32P]ATP and the plate is incubated at 25°C for 15 min. The reaction is terminated by addition of 0.1 mL of 20% trichloroacetic acid and the plate is kept at 4°C for at least 1 hour to allow the substrate to precipitate. The wells are then washed 5 times with 0.2 mL of 10% trichloroacetic acid and radioactive incorporation is determined with a β plate counter. |
| Cell Assay | MRT cell lines, G401, MP-MRT-AN (AN), KP-MRT-RY (RY), KP-MRT-NS (NS), and KP-MRT-YM (YM) cell lines are seeded in normal growth medium into 96-well cell plates. After 24 h, the culture medium is replaced with culture medium containing Palbociclib (0.05 or 1 µM) or DMSO. Cells are cultured and treated in triplicate. Cell proliferation is determined 8 days after the treatment by WST-8 assay using a Cell Counting Kit-8. |
| Animal Admin | Mice (18-22 g) are randomized and then implanted s.c. with tumor fragments (30 mg) into the region of the right axilla. Treatment is initiated when tumors reach 100 to 150 mg. PD 0332991 (150 or 75 mg/kg, p.o.) is given according to the schedule and dose indicated in the table and figure legends by gavage as a solution in sodium lactate buffer (50 mM, pH 4.0) based on mean group body weight. In all experiments, there are 12 mice in the control group and 8 mice each in the treated groups. Additional details for each experiment are given in the table legends. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 711.5±70.0 °C at 760 mmHg |
| Melting Point | 200ºC |
| Molecular Formula | C24H29N7O2 |
| Molecular Weight | 447.533 |
| Flash Point | 384.1±35.7 °C |
| Exact Mass | 447.238281 |
| PSA | 105.04000 |
| LogP | 0.99 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.648 |
| Hazard Codes | T+ |
|---|---|
| RIDADR | 2811.0 |
|
~% 
palbociclib CAS#:571190-30-2 |
| Literature: WO2005/5426 A1, ; Page/Page column 22 ; WO 2005/005426 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| PD0332991 |
| PD332991 |
| 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one |
| Pyrido[2,3-d]pyrimidin-7(8H)-one, 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]- |
| Palbociclib |
| 6-Acetyl-8-cyclopentyl-5-methyl-2-{[5-(1-piperazinyl)-2-pyridinyl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one |
| UNII-G9ZF61LE7G |
| PD 0332991 |
| 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8H-pyrido(2,3-d)pyrimidin-7-one |
| Ibrance |