Cosmosiin

Cosmosiin structure
|
Common Name | Cosmosiin | ||
|---|---|---|---|---|
| CAS Number | 578-74-5 | Molecular Weight | 432.378 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 788.9±60.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O10 | Melting Point | 230-237ºC | |
| MSDS | Chinese USA | Flash Point | 280.7±26.4 °C | |
Use of CosmosiinApigenin-7-glucoside exhibits significant anti-proliferative and antioxidant activity, scavengers of ROS.In vitro: exhibits significant anti-proliferative activity against B16F10 melanoma cells after 24 and 48 h of incubation. Apigenin-7-glucoside provoks an increase of subG0/G1, S and G2/M phase cell proportion with a significant decrease of cell proportion in G0/G1 phases. Apigenin-7-glucoside enhances melanogenesis synthesis and tyrosinase activity of B16F10 melanoma cells. [1] Api7G specifically induced the differentiation of CD34+ cells towards the erythroid lineage and inhibited the myeloid differentiation. [2] APIG had strong antioxidant activity against reactive oxygen species (ROS) in vitro in a concentration-dependent manner. |
| Name | apigenin 7-O-β-D-glucoside |
|---|---|
| Synonym | More Synonyms |
| Description | Apigenin-7-glucoside exhibits significant anti-proliferative and antioxidant activity, scavengers of ROS.In vitro: exhibits significant anti-proliferative activity against B16F10 melanoma cells after 24 and 48 h of incubation. Apigenin-7-glucoside provoks an increase of subG0/G1, S and G2/M phase cell proportion with a significant decrease of cell proportion in G0/G1 phases. Apigenin-7-glucoside enhances melanogenesis synthesis and tyrosinase activity of B16F10 melanoma cells. [1] Api7G specifically induced the differentiation of CD34+ cells towards the erythroid lineage and inhibited the myeloid differentiation. [2] APIG had strong antioxidant activity against reactive oxygen species (ROS) in vitro in a concentration-dependent manner. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 788.9±60.0 °C at 760 mmHg |
| Melting Point | 230-237ºC |
| Molecular Formula | C21H20O10 |
| Molecular Weight | 432.378 |
| Flash Point | 280.7±26.4 °C |
| Exact Mass | 432.105652 |
| PSA | 170.05000 |
| LogP | -0.39 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.717 |
| InChIKey | KMOUJOKENFFTPU-QNDFHXLGSA-N |
| SMILES | O=c1cc(-c2ccc(O)cc2)oc2cc(OC3OC(CO)C(O)C(O)C3O)cc(O)c12 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 0 | |
|---|---|
| DownStream 3 | |
|
Mesophyll distribution of 'antioxidant' flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance.
Ann. Bot. 104(5) , 853-61, (2009) Flavonoids have the potential to serve as antioxidants in addition to their function of UV screening in photoprotective mechanisms. However, flavonoids have long been reported to accumulate mostly in ... |
|
|
Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé
Food Chem. Toxicol. 49(12) , 3328-35, (2011) Highlights ► We present here the purification of 13 compounds from an MeOH extract of Marrubium deserti. ► We measure antioxidant, genotoxic and antigenotoxic activites of six phenolic compounds. ► Tw... |
|
|
Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids.
Bioorg. Med. Chem. 18 , 28-35, (2010) The applicability of the newly developed RM1 and PM6 methods implemented in the semiempirical quantum chemistry mopac2009 software package in modeling free radical scavenging activity of flavonoids wa... |
| Apigenin-7-O-Beta-D-glucopyranoside |
| 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxyphenyl)- |
| Apigenin 7-β-glucoside |
| Cosmosiin |
| MFCD00016787 |
| Cosmosioside |
| Apigenin 7-glucoside |
| Apigenin-7-O-β-D-glucopyranoside |
| Cosmosiin (8CI) |
| Apigenin 7-β-D-glucoside |
| Cosmiin |
| Cosmosin |
| Thalictiin |
| apigenin 7-O-beta-D-glucoside |
| Apigenin-7-O-glucoside |
| Apigetrin |
| COSMETIN |
| Cosemetin |
| EINECS 209-430-5 |
| 7-D-Glycosylapigenin |
| 5-Hydroxy-2-(4-hydroxyphényl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one |
| 5-Hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl b-D-glucopyranoside |
| Apigenin 7-O-β-D-glucopyranoside |
| 5-Hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl-β-D-glucopyranoside |
| 5-Hydroxy-2-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on |
| 7-(b-D-Glucopyranosyloxy)-5-hydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one |
| Cossmetin |
| 5-Hydroxy-2-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one |
| Apigenin-7-d-glucoside |
| Apigenin 7-O-β-glucoside |
| Apigenin 7-β-D-glucopyranoside |
| 5-Hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl β-D-glucopyranoside |
| Apigetrin,Cosmosiin |
| Apigenin 7-O-glucoside |
| 4H-1-Benzopyran-4-one, 7- (β-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxyphenyl)- |
| Apigenin-7-glucoside |
| Apigenin-7-O-β-glucopyranoside |
| apigenin 7-O-β-D-glucoside |
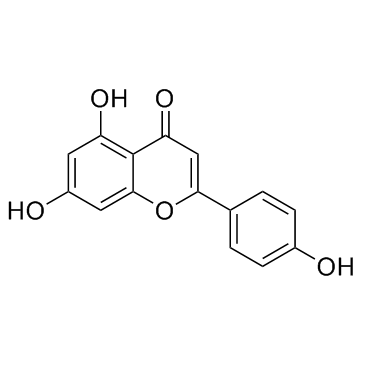 CAS#:520-36-5
CAS#:520-36-5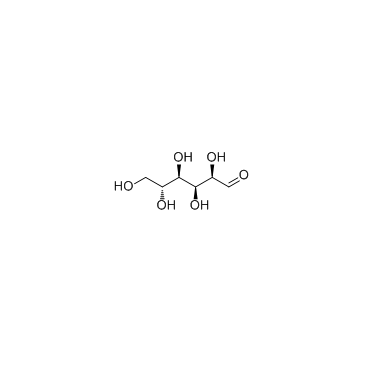 CAS#:50-99-7
CAS#:50-99-7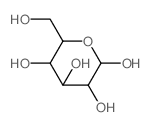 CAS#:2280-44-6
CAS#:2280-44-6
