Stylomycin aminonucleoside
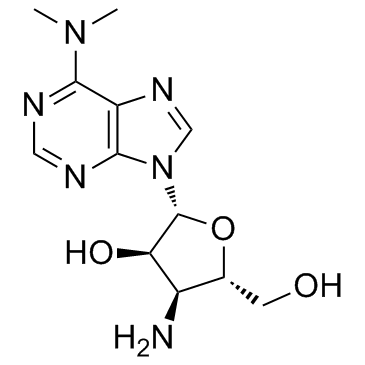
Stylomycin aminonucleoside structure
|
Common Name | Stylomycin aminonucleoside | ||
|---|---|---|---|---|
| CAS Number | 58-60-6 | Molecular Weight | 294.310 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 595.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C12H18N6O3 | Melting Point | 235℃ (Decomposition) | |
| MSDS | Chinese USA | Flash Point | 314.0±32.9 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of Stylomycin aminonucleosidePuromycin aminonucleoside is the aminonucleoside portion of the antibiotic puromycin, and a puromycin analog which does not inhibit protein synthesis or induce apoptosis. |
| Name | 3'-amino-3'-deoxy-N6,N6-dimethyladenosine |
|---|---|
| Synonym | More Synonyms |
| Description | Puromycin aminonucleoside is the aminonucleoside portion of the antibiotic puromycin, and a puromycin analog which does not inhibit protein synthesis or induce apoptosis. |
|---|---|
| Related Catalog | |
| In Vitro | Puromycin aminonucleoside (30 μg/mL) markedly increases p53 protein levels in podocytes. Puromycin aminonucleoside-induced podocyte apoptosis is p53 dependent and supports the notion that dexamethasone exerts an antiapoptotic effect on cells that are exposed to Puromycin aminonucleoside through the downregulation of p53. Puromycin aminonucleoside induces podocyte apoptosis in a time-dependent manner[1]. The IC50 values for PMAT-expressing and vector-transfected cells are 48.9 and 122.1 μM, respectively, suggesting expression of PMAT-enhanced cell sensitivity to Puromycin aminonucleoside. Puromycin aminonucleoside (250 μM) is toxic to both PMAT-expressing and vector-transfected cells. Puromycin aminonucleoside uptake in PMAT-expressing cells is fourfold higher at pH 6.6 than that at pH 7.4[2]. |
| In Vivo | The number of podocytes per glomerulus is 95.5±17.6 in the control rats, 90.7 on Day 4 in Puromycin aminonucleoside (8 mg/100 g, i.v.)-treated nephrosis rats. The amount of nephrin per glomerulus in control rats is 1.02±0.11 fmol and those in Puromycin aminonucleoside nephrosis rats are reduced to 0.46±0.06 fmol and 0.35±0.04 fmol on Day 4 and Day 7. The nephrin amount per podocyte is significantly decreased association with the development of proteinuria in Puromycin aminonucleoside nephrosis rats[3]. Rats given Puromycin aminonucleoside (100 mg/kg, s.c.) gain less weight and their serum creatinine levels are higher than the control rats, indicating imPuromycin aminonucleosideired renal function[4]. |
| Cell Assay | Cells are seeded in MEM with 10% FBS on 96-well plates at a density of 5,000 cells/well. After appr 48-h incubation (appr 40-50% confluence), cells are changed to fresh growth medium containing Puromycin aminonucleoside at various concentrations. For the protection experiment, cells are incubated in medium containing 250 μM Puromycin aminonucleoside with or without the PMAT inhibitor decynium-22 (2 μM). After a total of 72-h incubation in a 95% O2 incubator at 37°C, cells are washed and the plates. The IC50 values are determined by fitting the cell growth data to the following model using nonlinear regression (WinNonLin version 3.2): S=Smax − [Smax − S0] × [Cγ/(Cγ + IC50γ)], where S is the cell survival expressed as percentage of the optical density to untreated control cells, Smax is the maximal cell survival, S0 is the lowest residual cell survival at the high drug concentration, C is Puromycin aminonucleoside concentration, γ is the Hill coefficient, and IC50 is the Puromycin aminonucleoside concentration leading to half-maximal cell survival. Five to six determinations are carried out within each experiment, and four independent experiments are performed. |
| Animal Admin | Male F344 rats at 11 weeks of age are purchased from JaPuromycin aminonucleoside SLC. Normal rats and a Puromycin aminonucleoside nephrosis model are used in the present study. Puromycin aminonucleoside nephrosis is induced in rats by a single intravenous injection of Puromycin aminonucleoside at a dose of 8 mg/100 g body weight in saline. Control animals receive an identical volume of saline. Nephrotic rats (n=6 per group) are studied at Days 4 and 7 after the Puromycin aminonucleoside injection. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 595.6±60.0 °C at 760 mmHg |
| Melting Point | 235℃ (Decomposition) |
| Molecular Formula | C12H18N6O3 |
| Molecular Weight | 294.310 |
| Flash Point | 314.0±32.9 °C |
| Exact Mass | 294.144043 |
| PSA | 122.55000 |
| LogP | 0.02 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.776 |
| Water Solubility | H2O: 50 mg/mL, clear, slightly yellow | Soluble in water at 50mg/ml. May require heating |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
Diagnostic and predictive performance and standardized threshold of traditional biomarkers for drug-induced liver injury in rats.
J. Appl. Toxicol. 35(2) , 165-72, (2014) Traditional biomarkers such as alanine and aspartate aminotransferase (ALT, AST) and total bilirubin (TBIL) have been widely used for detecting drug-induced liver injury (DILI). Although the Food and ... |
|
|
Cyclosporine A protects podocytes by regulating WAVE1 phosphorylation.
Sci. Rep. 5 , 17694, (2015) Accumulating evidence suggests that podocytes are direct targets of many classic antiproteinuric drugs. The immunosuppressive drug cyclosporine A (CsA), which is a calcineurin inhibitor, is used to tr... |
|
|
Fluvastatin attenuated the effect of expression of β1 integrin in PAN-treated podocytes by inhibiting reactive oxygen species.
Mol. Cell Biochem. 398(1-2) , 207-15, (2014) It is well accepted that β1 integrin plays a key role in maintaining normal podocytes form and functions; however, its mechanism of the potential protective effect remains unclear. Furthermore, the in... |
| 3'-amino-3'-deoxy-N(6),N(6)-dimethyladenosine |
| EINECS 200-388-3 |
| MFCD00063462 |
| 9-(3-Amino-3-deoxy-β-D-ribofuranosyl)-N,N-dimethyl-9H-purin-6-amine |
| Adenosine, 3'-amino-3'-deoxy-N,N-dimethyl- |
| 3'-Amino-3'-deoxy-N,N-dimethyladenosine |
| PUROMYCIN AMINONUCLEOSIDE |
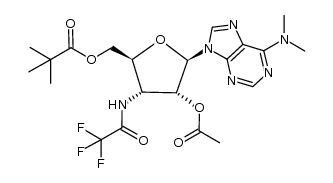 CAS#:178883-52-8
CAS#:178883-52-8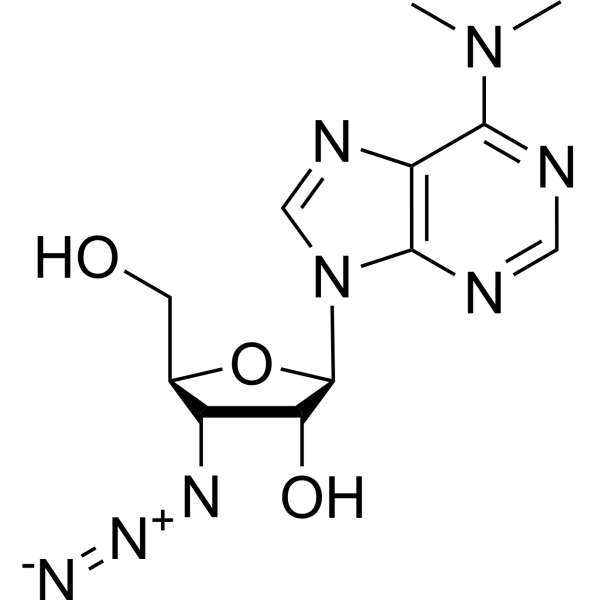 CAS#:384334-64-9
CAS#:384334-64-9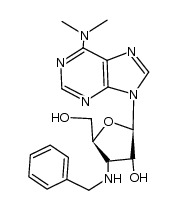 CAS#:36913-17-4
CAS#:36913-17-4 CAS#:53-79-2
CAS#:53-79-2
