Glycolophenone
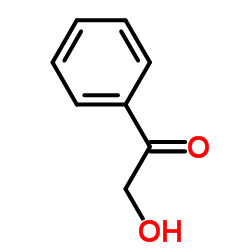
Glycolophenone structure
|
Common Name | Glycolophenone | ||
|---|---|---|---|---|
| CAS Number | 582-24-1 | Molecular Weight | 136.148 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 244.6±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O2 | Melting Point | 86-89 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 100.4±12.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Glycolophenone2-Hydroxyacetophenone is a principal root volatile of the Carissa edulis[1]. 2-Hydroxyacetophenone shows inhibitory effects on infection of HIV/SARS-CoV S pseudovirus with an IC50 of 1.8 mM[2]. |
| Name | 2-hydroxyacetophenone |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Hydroxyacetophenone is a principal root volatile of the Carissa edulis[1]. 2-Hydroxyacetophenone shows inhibitory effects on infection of HIV/SARS-CoV S pseudovirus with an IC50 of 1.8 mM[2]. |
|---|---|
| Related Catalog | |
| Target |
HIV |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 244.6±13.0 °C at 760 mmHg |
| Melting Point | 86-89 °C(lit.) |
| Molecular Formula | C8H8O2 |
| Molecular Weight | 136.148 |
| Flash Point | 100.4±12.4 °C |
| Exact Mass | 136.052429 |
| PSA | 37.30000 |
| LogP | 0.44 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.551 |
| InChIKey | ZWVHTXAYIKBMEE-UHFFFAOYSA-N |
| SMILES | O=C(CO)c1ccccc1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914400090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914400090 |
|---|---|
| Summary | 2914400090 other ketone-alcohols and ketone-aldehydes。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Urinalysis of minor metabolites of ethylbenzene and m-xylene.
Scand. J. Work. Environ. Health 10(2) , 75-81, (1984) Gas chromatographic methods have been developed for the urinalysis of metabolic products of ethylbenzene. "Minor" metabolites were emphasized in this process. The methods were worked out so that simul... |
|
|
Synthesis and structural characterization of mixed ligand ? 1-2-hydroxyacetophenone complexes of cobalt (III). Mondal N, et al.
Polyhedron 19(28) , 2707-11, (2000)
|
|
|
Solvent-free microwave-assisted Beckmann rearrangement of benzaldehyde and 2-hydroxyacetophenone oximes. Loupy A and Régnier S.
Tetrahedron Lett. 40(34) , 6221-24, (1999)
|
| 2-Hydroxyacetophenone |
| MFCD00041829 |
| Glycolophenone |
| EINECS 209-480-8 |
| Methanol, benzoyl- |
| Benzoylcarbinol |
| 2-Hydroxy-1-phenylethanone |
| HYDROXYACETOPHENONE |
| α-hydroxyacetophenone |
| Ethanone, 2-hydroxy-1-phenyl- |
 CAS#:70-11-1
CAS#:70-11-1 CAS#:13735-81-4
CAS#:13735-81-4 CAS#:96-09-3
CAS#:96-09-3 CAS#:292638-84-7
CAS#:292638-84-7 CAS#:93-56-1
CAS#:93-56-1 CAS#:1074-12-0
CAS#:1074-12-0 CAS#:50-00-0
CAS#:50-00-0 CAS#:100-52-7
CAS#:100-52-7 CAS#:2243-35-8
CAS#:2243-35-8 CAS#:4636-16-2
CAS#:4636-16-2 CAS#:3327-24-0
CAS#:3327-24-0 CAS#:347-91-1
CAS#:347-91-1 CAS#:557-40-4
CAS#:557-40-4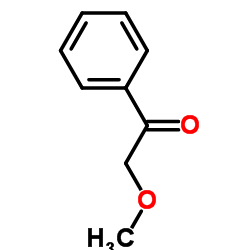 CAS#:4079-52-1
CAS#:4079-52-1 CAS#:5271-26-1
CAS#:5271-26-1 CAS#:93-89-0
CAS#:93-89-0 CAS#:98-85-1
CAS#:98-85-1 CAS#:17422-74-1
CAS#:17422-74-1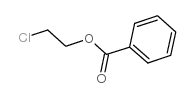 CAS#:939-55-9
CAS#:939-55-9
