| Description |
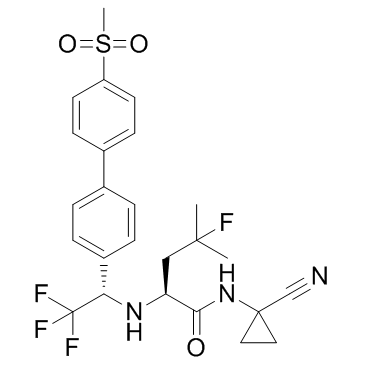
Odanacatib (MK-0822) is a potent and selective inhibitor of cathepsin K, with an IC50 of 0.2 nM for human cathepsin K.
|
| Related Catalog |
|
| Target |
IC50: 0.2 nM (Human Cathepsin K), 1 nM (Rabbit Cathepsin K)
|
| In Vitro |
Odanacatib is a weak inhibitor of antigen presentation, measured in a mouse B cell line (IC50=1.5±0.4 μM), compared to the Cat S inhibitor LHVS (IC50=0.001 μM) in the same assay. Odanacatib also shows weak inhibition of the processing of the MHC II invariant chain protein Iip10 in mouse splenocytes compared to LHVS (minimum inhibitory concentration 1-10 μM versus 0.01 μM, respectively)[1]. Odanacatib reduces resorption activity as measured by CTx release (IC50=9.4 nM) or resorption area (IC50=6.5 nM), but has no impact on OC activation. Odanacatib dose-dependently reduces CTx release with an IC50=9.4±1.0 nM. Odanacatib treated OC accumulates labeled degraded bone matrix proteins in CatK containing vesicles[2].
|
| In Vivo |
Odanacatib (30 mg/kg, orally, once daily) persistently suppresses bone resorption markers and serum bone formation markers versus vehicle-treated OVX monkeys. Odanacatib displays compartment-specific effects on trabecular versus cortical bone formation, with treatment resulting in marked increases in periosteal bone formation and cortical thickness in ovariectomized monkeys whereas trabecular bone formation is reduced[3]. The bone volume/total volume (BV/TV) and bone mineral density (BMD) of the OVX + ODN-h group is significantly higher than that of the OVX + Veh group (p < 0.05). The expressions of Runx2, Collagen-1, BSP, Osterix, OPN and SPP1 are significantly lower in the OVX + ODN-h group than in the OVX + Veh group (p < 0.01). Compared with the OVX + Veh group, the expressions of Collagen-I, BSP, Osterix, OPN and ALP reduce in the OVX + ODN-l group, but are upregulated in the OVX + ODN-h group[4].
|
| Cell Assay |
To assess cell survival, differentiated osteoclast (OC) at appr 7×104 cells/cm2 are re-seeded on bovine bone slices with or without 100 nM Odanacatib (ODN). Bone slices are fixed on days 2, 4, 6, and 12 with no media changes. Samples are stained for TRAP activity, and OC number.
|
| Animal Admin |
Sixteen, 8-month-old, female Sprague-Dawley (SD) rats (weight, 385 ± 55 g) are given water and soft diet food ad libitum in a temperature-controlled environment with regular 12-h cycles of light and dark. The rats are randomised into 4 groups, with 4 rats in each group: sham group, OVX + Veh group, OVX + ODN-l group and OVX + ODN-h group. Following implant insertion, Odanacatib (ODN, 5 mg/mL) is administered to the OVX + ODN-l and OVX + ODN-h groups at concentrations of 1 mL/kg and 6 mL/kg, respectively, by gavaging once a day for 8 weeks. The OVX + Veh group is gavaged with 0.5% sodium carboxymethyl cellulose at a concentration of 6 mL/kg over the same duration. After the gavage administration, the rats of each group are sacrificed by injecting sodium pentobarbital intravenously. The implants are harvested and fixed in 10% buffered formalin together with the surrounding bone.
|
| References |
[1]. Jacques Yves Gauthier, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008 Feb 1;18(3):923-8. [2]. Leung P, et al. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone. 2011 Oct;49(4):623-635. [3]. Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235-47. [4]. Yi C, et al. Inhibition of cathepsin K promotes osseointegration of titanium implants in ovariectomised rats. Sci Rep. 2017 Mar 17;7:44682.
|