2,4-Dihydroxy-5-nitropyrimidine
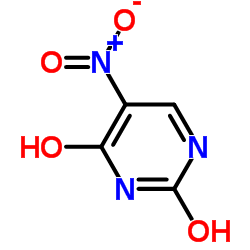
2,4-Dihydroxy-5-nitropyrimidine structure
|
Common Name | 2,4-Dihydroxy-5-nitropyrimidine | ||
|---|---|---|---|---|
| CAS Number | 611-08-5 | Molecular Weight | 157.084 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 453.3±48.0 °C at 760 mmHg | |
| Molecular Formula | C4H3N3O4 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 227.9±29.6 °C | |
Use of 2,4-Dihydroxy-5-nitropyrimidine |
| Name | 5-Nitrouracil |
|---|---|
| Synonym | More Synonyms |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 453.3±48.0 °C at 760 mmHg |
| Melting Point | >300 °C(lit.) |
| Molecular Formula | C4H3N3O4 |
| Molecular Weight | 157.084 |
| Flash Point | 227.9±29.6 °C |
| Exact Mass | 157.012360 |
| PSA | 111.54000 |
| LogP | -1.72 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.701 |
| InChIKey | TUARVSWVPPVUGS-UHFFFAOYSA-N |
| SMILES | O=c1[nH]cc([N+](=O)[O-])c(=O)[nH]1 |
| Storage condition | -20°C Freezer |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C: Corrosive;F: Flammable; |
| Risk Phrases | R11 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | UV9104545 |
| HS Code | 2933599090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Phenobarbital induction and chemical synergism demonstrate the role of UDP-glucuronosyltransferases in detoxification of naphthalophos by Haemonchus contortus larvae.
Antimicrob. Agents Chemother. 58(12) , 7475-83, (2014) We used an enzyme induction approach to study the role of detoxification enzymes in the interaction of the anthelmintic compound naphthalophos with Haemonchus contortus larvae. Larvae were treated wit... |
|
|
Inhibition of UDP-glucuronyltransferase activity in rat liver microsomes by pyrimidine derivatives.
Comp. Biochem. Physiol. C, Pharmacol. Toxicol. Endocrinol. 112(3) , 321-5, (1995) Thirty-one differently substituted pyrimidine bases were tested for their inhibitory effect on the glucuronidation of 4-nitrophenol and phenolphthalein by rat liver microsomes. 5-Nitrouracil (compound... |
|
|
Thymidine phosphorylase inhibitors with a homophthalimide skeleton.
Biol. Pharm. Bull. 24(7) , 860-2, (2001) Several N-phenylhomophthalimide derivatives were prepared and their inhibitory activity on thymidine phosphorylase/ platelet-derived endothelial cell growth factor (TP/PD-ECGF) was assessed. Among the... |
| 2,4(1H,3H)-Pyrimidinedione, 5-nitro- |
| 5-nitropyrimidine-2,4(1H,3H)-dione |
| 5-NITRO-2,4-PYRIMIDINEDIOL |
| 5-Nitro-2,4(1H,3H)-pyrimidinedione |
| 5-nitro-1H-pyrimidine-2,4-dione |
| 5-nitropyrimidine-2,4-diol |
| MFCD00006021 |
| 2,4-Dihydroxy-5-Nitropyrimidine |
| 5-Nitro-1H-pyrimidin-2,4-dion |
| 5-Nitropyrimidin-2,4(1H,3H)-dion |
| NITROURACIL |
| Uracil,5-nitro |
| 4(1H)-pyrimidinone, 2-hydroxy-5-nitro- |
| 2-Hydroxy-5-nitropyrimidin-4(1H)-one |
| 5-nitro-uracil |
| EINECS 210-250-4 |
| 5-NITROURACIL extrapure |
 CAS#:66-22-8
CAS#:66-22-8 CAS#:626-48-2
CAS#:626-48-2 CAS#:6965-19-1
CAS#:6965-19-1 CAS#:141-90-2
CAS#:141-90-2 CAS#:49845-33-2
CAS#:49845-33-2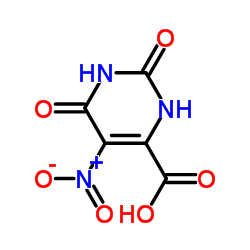 CAS#:17687-24-0
CAS#:17687-24-0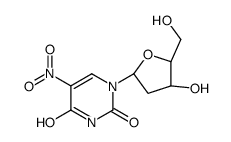 CAS#:3106-01-2
CAS#:3106-01-2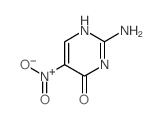 CAS#:7254-29-7
CAS#:7254-29-7 CAS#:7664-93-9
CAS#:7664-93-9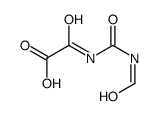 CAS#:106055-61-2
CAS#:106055-61-2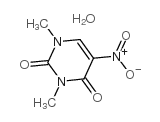 CAS#:41613-26-7
CAS#:41613-26-7 CAS#:28495-88-7
CAS#:28495-88-7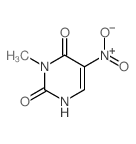 CAS#:25912-37-2
CAS#:25912-37-2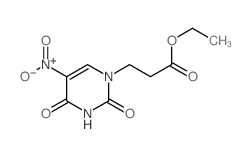 CAS#:2950-88-1
CAS#:2950-88-1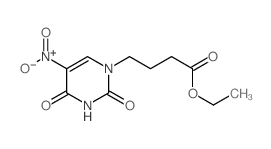 CAS#:2950-89-2
CAS#:2950-89-2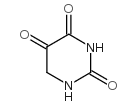 CAS#:20636-41-3
CAS#:20636-41-3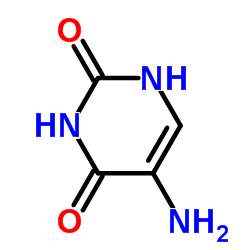 CAS#:932-52-5
CAS#:932-52-5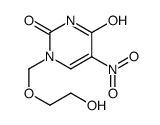 CAS#:81797-05-9
CAS#:81797-05-9
