Amoxicillin Trihydrate
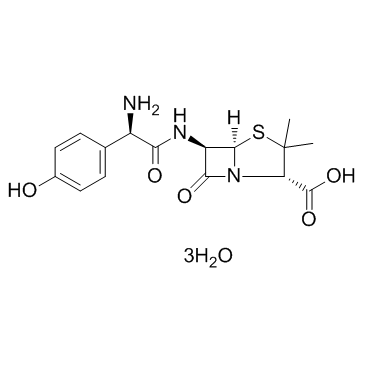
Amoxicillin Trihydrate structure
|
Common Name | Amoxicillin Trihydrate | ||
|---|---|---|---|---|
| CAS Number | 61336-70-7 | Molecular Weight | 419.450 | |
| Density | 1.54g/cm3 | Boiling Point | 743.2ºC at 760 mmHg Vapour | |
| Molecular Formula | C16H25N3O8S | Melting Point | >200ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 403.3ºC | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of Amoxicillin TrihydrateAmoxicillin Trihydrate is a moderate- spectrum, bacteriolytic, β-lactam antibiotic.Target: AntibacterialAmoxicillin is a moderate-spectrum, bacteriolytic, β-lactam antibiotic in the aminopenicillin family used to treat bacterial infections caused by susceptible Gram-positive and Gram-negative microorganisms. It is usually the drug of choice within the class because it is better-absorbed, following oral administration, than other β-lactam antibiotics. Amoxicillin is susceptible to degradation by β-lactamase-producing bacteria, which are resistant to a narrow spectrum of β-lactam antibiotics, such as penicillin. For this reason, it is often combined with clavulanic acid, a β-lactamase inhibitor. This increases effectiveness by reducing its susceptibility to β-lactamase resistance. From Wikipedia. |
| Name | amoxicillin trihydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Amoxicillin Trihydrate is a moderate- spectrum, bacteriolytic, β-lactam antibiotic.Target: AntibacterialAmoxicillin is a moderate-spectrum, bacteriolytic, β-lactam antibiotic in the aminopenicillin family used to treat bacterial infections caused by susceptible Gram-positive and Gram-negative microorganisms. It is usually the drug of choice within the class because it is better-absorbed, following oral administration, than other β-lactam antibiotics. Amoxicillin is susceptible to degradation by β-lactamase-producing bacteria, which are resistant to a narrow spectrum of β-lactam antibiotics, such as penicillin. For this reason, it is often combined with clavulanic acid, a β-lactamase inhibitor. This increases effectiveness by reducing its susceptibility to β-lactamase resistance. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.54g/cm3 |
|---|---|
| Boiling Point | 743.2ºC at 760 mmHg Vapour |
| Melting Point | >200ºC (dec.) |
| Molecular Formula | C16H25N3O8S |
| Molecular Weight | 419.450 |
| Flash Point | 403.3ºC |
| Exact Mass | 419.136230 |
| PSA | 185.95000 |
| LogP | 0.85990 |
| Index of Refraction | 302 ° (C=0.1, H2O) |
| InChIKey | AIPFZZHNBUVELL-YWUHCJSESA-N |
| SMILES | CC1(C)SC2C(NC(=O)C(N)c3ccc(O)cc3)C(=O)N2C1C(=O)O.O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R42/43 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| RTECS | XH8300000 |
| HS Code | 2941109200 |
| HS Code | 2941109200 |
|---|
|
Experimental study of the impact of antimicrobial treatments on Campylobacter, Enterococcus and PCR-capillary electrophoresis single-strand conformation polymorphism profiles of the gut microbiota of chickens.
J. Med. Microbiol. 63(Pt 11) , 1552-60, (2014) An experiment was conducted to compare the impact of antimicrobial treatments on the susceptibility of Campylobacter, Enterococcus faecium and Enterococcus faecalis, and on the diversity of broiler mi... |
|
|
Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family.
Molecules 19(12) , 20034-53, (2014) Six essential oils (EOs) from the Alliaceae family, namely garlic (Allium sativum), onion (Allium cepa), leek (Allium porrum), Chinese chive (Allium tuberosum), shallot (Allium ascalonicum) and chive ... |
|
|
Simultaneous determination of 38 veterinary antibiotic residues in raw milk by UPLC-MS/MS.
Food Chem. 181 , 119-26, (2015) A selective and rapid method has been developed to determine, simultaneously, 38 veterinary antibiotic residues in raw milk by ultra-high-performance liquid chromatography-tandem mass spectrometry (UP... |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-, hydrate (1:3) |
| (2S,5R,6R)-6-{[(2R)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate |
| Amoxicillin Trihydrate |
| Amoxycillin trihydrate |
| MFCD00072029 |
| AmoxicillinTrihydrate |
| Amoxicillin (trihydrate) |

