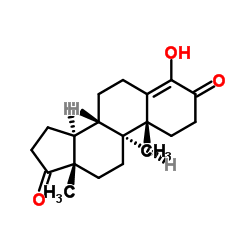
4-Androsten-4-ol-3,17-dione acetate
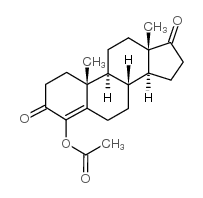
4-Androsten-4-ol-3,17-dione acetate structure
|
Common Name | 4-Androsten-4-ol-3,17-dione acetate | ||
|---|---|---|---|---|
| CAS Number | 61630-32-8 | Molecular Weight | 344.44500 | |
| Density | 1.18g/cm3 | Boiling Point | 501.2ºC at 760 mmHg | |
| Molecular Formula | C21H28O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 219.2ºC | |
| Name | 4-Androsten-4-ol-3,17-dione acetate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.18g/cm3 |
|---|---|
| Boiling Point | 501.2ºC at 760 mmHg |
| Molecular Formula | C21H28O4 |
| Molecular Weight | 344.44500 |
| Flash Point | 219.2ºC |
| Exact Mass | 344.19900 |
| PSA | 60.44000 |
| LogP | 3.97820 |
| Index of Refraction | 1.548 |
| InChIKey | LRXSFNGKRCOHRS-VMRCMBGLSA-N |
| SMILES | CC(=O)OC1=C2CCC3C4CCC(=O)C4(C)CCC3C2(C)CCC1=O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | BV8153000 |
|
Aromatase inhibitors and the treatment of breast cancer.
J. Steroid Biochem. 24 , 91, (1986) Inhibition of aromatase to reduce estrogen production by peripheral and ovarian tissue could be a useful approach to treating hormone-dependent breast cancer. Several C19, 17 keto steroids have been i... |
|
|
Inhibition of estrogen biosynthesis and regression of mammary tumors by aromatase inhibitors.
Adv. Exp. Med. Biol. 138 , 179-90, (1981)
|
|
|
Overview of recent development of aromatase inhibitors.
Cancer Res. 42(8 Suppl) , 3312s-3314s, (1982) Since the first publication in 1973 concerning aromatase inhibitors, several effective compounds have been reported by a number of investigators. Our studies with 4-hydroxyandrostene-3,17-dione, 4-ace... |
| [(8R,9S,10R,13S,14S)-10,13-dimethyl-3,17-dioxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-4-yl] acetate |