6-HYDROXYDOPAMINE HYDROBROMIDE
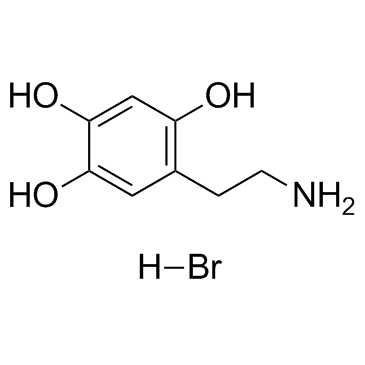
6-HYDROXYDOPAMINE HYDROBROMIDE structure
|
Common Name | 6-HYDROXYDOPAMINE HYDROBROMIDE | ||
|---|---|---|---|---|
| CAS Number | 636-00-0 | Molecular Weight | 250.090 | |
| Density | N/A | Boiling Point | 406ºC at 760 mmHg | |
| Molecular Formula | C8H12BrNO3 | Melting Point | 216-220 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 199.3ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 6-HYDROXYDOPAMINE HYDROBROMIDEOxidopamine hydrobromide is a selective catecholaminergic neurotoxin, depletes brain catecholamine levels via uptake and accumulation by a transport mechanism specific to these neurons. In vitro: Oxidopamine hydrobromide-induced apoptosis of PC12 cells was initiated by superoxide generation followed by caspase cascade activation, which was associated with the suppressed Akt phosphorylation and increased p38 phosphorylation. It is likely that pCPT-cAMP prevented the Oxidopamine hydrobromide-induced apoptosis via activation of the PI3-kinase/Akt pathway without any effect on superoxide generation or mitochondrial membrane depolarization. [1]In vivo the presence of sulfhydryl antioxidants protected against neuronal degeneration in the striatum, which was particularly remarkable in the case of CySH and was attributed to its capacity to remove the H2O2 produced in the autoxidation of Oxidopamine hydrobromide. |
| Name | 6-Hydroxydopamine hydrobromide |
|---|---|
| Synonym | More Synonyms |
| Description | Oxidopamine hydrobromide is a selective catecholaminergic neurotoxin, depletes brain catecholamine levels via uptake and accumulation by a transport mechanism specific to these neurons. In vitro: Oxidopamine hydrobromide-induced apoptosis of PC12 cells was initiated by superoxide generation followed by caspase cascade activation, which was associated with the suppressed Akt phosphorylation and increased p38 phosphorylation. It is likely that pCPT-cAMP prevented the Oxidopamine hydrobromide-induced apoptosis via activation of the PI3-kinase/Akt pathway without any effect on superoxide generation or mitochondrial membrane depolarization. [1]In vivo the presence of sulfhydryl antioxidants protected against neuronal degeneration in the striatum, which was particularly remarkable in the case of CySH and was attributed to its capacity to remove the H2O2 produced in the autoxidation of Oxidopamine hydrobromide. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 406ºC at 760 mmHg |
|---|---|
| Melting Point | 216-220 °C(lit.) |
| Molecular Formula | C8H12BrNO3 |
| Molecular Weight | 250.090 |
| Flash Point | 199.3ºC |
| Exact Mass | 249.000046 |
| PSA | 86.71000 |
| LogP | 1.96300 |
| Appearance of Characters | solid | tan to brown |
| InChIKey | MLACDGUOKDOLGC-UHFFFAOYSA-N |
| SMILES | Br.NCCc1cc(O)c(O)cc1O |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DC4600000 |
| HS Code | 2922299090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Rho kinase inhibition by fasudil in the striatal 6-hydroxydopamine lesion mouse model of Parkinson disease.
J. Neuropathol. Exp. Neurol. 73(8) , 770-9, (2014) Chronic degeneration of nigrostriatal projections, followed by nigral dopaminergic cell death, is a key feature of Parkinson disease (PD). This study examines the neuroprotective potential of the rho ... |
|
|
Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells.
Phytother Res. 28(4) , 611-6, (2014) 6-Hydroxydopamine (6-OHDA) selectively enters dopaminergic neurons and undergoes auto-oxidation resulting in the generation of reactive oxygen species and dopamine quinones, subsequently leading to ap... |
|
|
Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice.
Eur. Rev. Med. Pharmacol. Sci. 17(10) , 1360-8, (2013) Inflammation and oxidative stress are believed to contribute to neuronal degeneration of the nigrostriatal dopaminergic (DA) pathway in Parkinson's disease. Curcumin, a component of the yellow curry s... |
| 5-(2-Aminoethyl)-1,2,4-benzenetriol hydrobromide (1:1) |
| 1,2,4-Benzenetriol, 5-(2-aminoethyl)-, hydrobromide (1:1) |
| 5-(2-aminoethyl)benzene-1,2,4-triol,hydrobromide |
| 5-(2-Aminoethyl)benzene-1,2,4-triol hydrobromide (1:1) |
| EINECS 211-247-0 |
| MFCD00012894 |
| Oxidopamine (hydrobromide) |
 CAS#:3131-52-0
CAS#:3131-52-0