Ketoconazole

Ketoconazole structure
|
Common Name | Ketoconazole | ||
|---|---|---|---|---|
| CAS Number | 65277-42-1 | Molecular Weight | 531.431 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 753.4±60.0 °C at 760 mmHg | |
| Molecular Formula | C26H28Cl2N4O4 | Melting Point | 146°C | |
| MSDS | Chinese USA | Flash Point | 409.4±32.9 °C | |
| Symbol |



GHS06, GHS08, GHS09 |
Signal Word | Danger | |
Use of KetoconazoleKetoconazole is an imidazole anti-fungal agent, a CYP3A4 and CYP24A1 inhibitor.Target: CYP3A4 CYP24A1Ketoconazole, an imidazole anti-fungal agent, has often produced features of androgen deficiency including decreased libido, gynecomastia, impotence, oligospermia, and decreased testosterone levels, in men being treated for chronic mycotic infections [1]. Ketoconazole also is a cytochrome P450 inhibitor [2].Ketoconazole (KTZ), on the antischistosomal potential of these quinolines against Schistosoma mansoni infection by evaluating parasitological, histopathological, and biochemical parameters. Mice were classified into 7 groups: uninfected untreated (I), infected untreated (II), infected treated orally with PZQ (1,000 mg/kg) (III), QN (400 mg/kg) (IV), KTZ (10 mg/kg)+QN as group IV (V), HF (400 mg/kg) (VI), and KTZ (as group V)+HF (as group VI) (VII). KTZ plus QN or HF produced more inhibition (P<0.05) in hepatic CYP450 (85.7% and 83.8%) and CYT b5 (75.5% and 73.5%) activities, respectively, than in groups treated with QN or HF alone. This was accompanied with more reduction in female (89.0% and 79.3%), total worms (81.4% and 70.3%), and eggs burden (hepatic; 83.8%, 66.0% and intestinal; 68%, 64.5%), respectively, and encountering the granulomatous reaction to parasite eggs trapped in the liver.[3] CYP24A1 inhibitor enhances antiproliferative effects, increases systemic calcitriol exposure, and promotes the activation of caspase-independent apoptosis pathway.[4] |
| Name | Ketoconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Ketoconazole is an imidazole anti-fungal agent, a CYP3A4 and CYP24A1 inhibitor.Target: CYP3A4 CYP24A1Ketoconazole, an imidazole anti-fungal agent, has often produced features of androgen deficiency including decreased libido, gynecomastia, impotence, oligospermia, and decreased testosterone levels, in men being treated for chronic mycotic infections [1]. Ketoconazole also is a cytochrome P450 inhibitor [2].Ketoconazole (KTZ), on the antischistosomal potential of these quinolines against Schistosoma mansoni infection by evaluating parasitological, histopathological, and biochemical parameters. Mice were classified into 7 groups: uninfected untreated (I), infected untreated (II), infected treated orally with PZQ (1,000 mg/kg) (III), QN (400 mg/kg) (IV), KTZ (10 mg/kg)+QN as group IV (V), HF (400 mg/kg) (VI), and KTZ (as group V)+HF (as group VI) (VII). KTZ plus QN or HF produced more inhibition (P<0.05) in hepatic CYP450 (85.7% and 83.8%) and CYT b5 (75.5% and 73.5%) activities, respectively, than in groups treated with QN or HF alone. This was accompanied with more reduction in female (89.0% and 79.3%), total worms (81.4% and 70.3%), and eggs burden (hepatic; 83.8%, 66.0% and intestinal; 68%, 64.5%), respectively, and encountering the granulomatous reaction to parasite eggs trapped in the liver.[3] CYP24A1 inhibitor enhances antiproliferative effects, increases systemic calcitriol exposure, and promotes the activation of caspase-independent apoptosis pathway.[4] |
|---|---|
| Related Catalog | |
| References |
[2]. Eil C. Ketoconazole binds to the human androgen receptor. Horm Metab Res. 1992 Aug;24(8):367-70. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 753.4±60.0 °C at 760 mmHg |
| Melting Point | 146°C |
| Molecular Formula | C26H28Cl2N4O4 |
| Molecular Weight | 531.431 |
| Flash Point | 409.4±32.9 °C |
| Exact Mass | 530.148743 |
| PSA | 69.06000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.642 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS06, GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H360F-H373-H410 |
| Precautionary Statements | P201-P273-P301 + P310-P308 + P313-P501 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S36-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | TK7912300 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~53% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 35, # 15 p. 2818 - 2825 |
|
~% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 16, # 4 p. 887 - 890 |
|
~% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 16, # 4 p. 887 - 890 |
|
~% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 16, # 4 p. 887 - 890 |
|
~% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 16, # 4 p. 887 - 890 |
|
~% 
Ketoconazole CAS#:65277-42-1 |
| Literature: Tetrahedron: Asymmetry, , vol. 6, # 6 p. 1283 - 1294 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites.
Arch. Toxicol. 89(1) , 107-19, (2015) The use of diclofenac (DCF), a nonsteroidal anti-inflammatory drug, is associated with a high prevalence of gastrointestinal side effects. In vivo studies in rodents suggested that reactive metabolite... |
|
|
Neuropharmacokinetics of two investigational compounds in rats: divergent temporal profiles in the brain and cerebrospinal fluid.
Biochem. Pharmacol. 91(4) , 543-51, (2014) Two investigational compounds (FRM-1, (R)-7-fluoro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide and FRM-2, (R)-7-cyano-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide) resided in rat brain ... |
|
|
Activation of the pleiotropic drug resistance pathway can promote mitochondrial DNA retention by fusion-defective mitochondria in Saccharomyces cerevisiae.
G3 (Bethesda) 4(7) , 1247-58, (2014) Genetic and microscopic approaches using Saccharomyces cerevisiae have identified many proteins that play a role in mitochondrial dynamics, but it is possible that other proteins and pathways that pla... |
| (2R,4S)-ketoconazole |
| Brizoral |
| Ketoconazole |
| Onofin K |
| Nizral |
| Ethanone, 1-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]- |
| 1-[4-(4-{[(4S)-2-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethanone |
| Fitonal |
| 1-[4-(4-{[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)-1-piperazinyl]ethanone |
| MFCD00058579 |
| Fungarest |
| EINECS 265-667-4 |
| 1-[4-(4-{[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethanone |
| piperazine, 1-acetyl-4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]- |
| 1-Acetyl-4-(4-{[(2R,4S)-2-(2,4-dichlorphenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin |
| cis-1-Acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazine |
| Fungoral |
| Ketoisdin |
| PANFUNGOL |
| Ketoderm |
| kw-1414 |
| NIZORAL |
| Orifungal M |
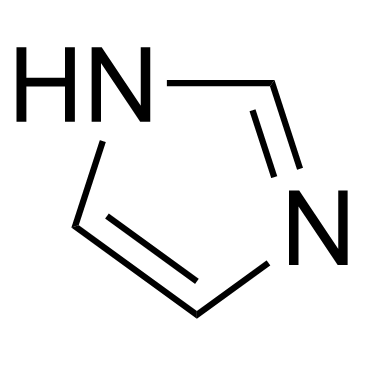

![(2R,4S)-cis-2-(hydroxymethyl)-2-(2,4-dichlorophenyl)-4-[[4-(4-acetylpiperazin-1-yl)phenoxy]methyl]-1,3-dioxolane structure](https://image.chemsrc.com/caspic/143/142004-25-9.png)
![(2R,4S)-(+)-2-(2,4-dichlorophenyl)-2-[(1H-imidazol-1-yl)methyl]-1,3-dioxolane-4-methanol structure](https://image.chemsrc.com/caspic/400/170210-42-1.png)

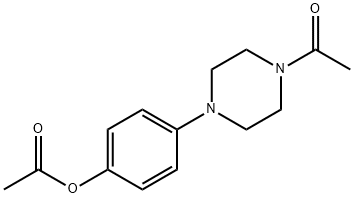


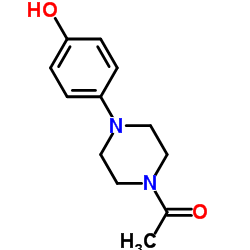
 CAS#:38304-91-5
CAS#:38304-91-5
