Ginsenoside Rb3
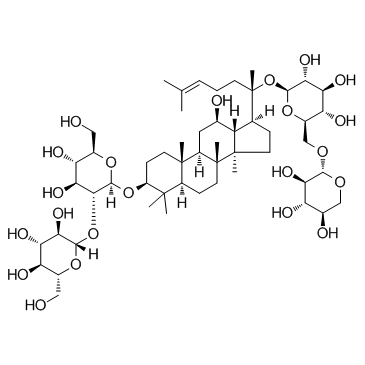
Ginsenoside Rb3 structure
|
Common Name | Ginsenoside Rb3 | ||
|---|---|---|---|---|
| CAS Number | 68406-26-8 | Molecular Weight | 1079.269 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 1117.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C53H90O22 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 629.4±34.3 °C | |
Use of Ginsenoside Rb3Ginsenoside Rb3 is extracted from steamed Panax notoginseng. Ginsenoside Rb3 exhibits inhibitory effect on TNFα-induced NF-κB transcriptional activity with an IC50 of 8.2 μM in 293T cell lines. Ginsenoside Rb3 also inhibits the induction of COX-2 and iNOS mRNA. |
| Name | ginsenoside Rb3 |
|---|---|
| Synonym | More Synonyms |
| Description | Ginsenoside Rb3 is extracted from steamed Panax notoginseng. Ginsenoside Rb3 exhibits inhibitory effect on TNFα-induced NF-κB transcriptional activity with an IC50 of 8.2 μM in 293T cell lines. Ginsenoside Rb3 also inhibits the induction of COX-2 and iNOS mRNA. |
|---|---|
| Related Catalog | |
| Target |
NF-κB:8.2 μM (IC50, in 293T cell lines) COX-2 iNOS |
| In Vitro | Ginsenoside Rb3 (0.1-10 μM) is tested for inhibition of tumor necrosis factor-α (TNF)-induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) luciferase reporter activity using a human kidney 293T cell-based assay. Ginsenoside Rb3 shows the significant activity with an IC50 of 8.2 μM. Ginsenoside Rb3 also inhibits the induction of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) messenger Ribonucleic acid (mRNA) in a dose-dependent manner after HepG2 cells have been treated with TNF-α (10 ng/mL)[1]. Ginsenoside Rb3 (0.1-10 μM) significantly increases cell viability and inhibits lactate dehydrogenase (LDH) release in a dose-dependent manner. PC12 cell viability as determined by MTT reduction is also markedly decreased after the cell is exposed to oxygen and glucose deprivation (OGD)/OGD-Rep. But, when the cells are pretreated with Ginsenoside Rb3 (0.1, 1, and 10 μM), OGD/OGD-Rep induced cell toxicity is significantly attenuated, which is concentration-dependently attenuated by Ginsenoside Rb3 treatment. The viabilities are raised to 52.8%±5.6%, 64.6%±5.7%, and 76.4%±8.8%, respectively, compared with the control group[2]. |
| In Vivo | Ginsenosides Rb3 is a major compound isolated from Gynostemma pentaphyllum that holistically improves gut microenvironment and induces anti-polyposis in ApcMin/+ mice. Six-weeks-old mice are subjected to Rb3 treatment, before the appearance of the intestinal polyps. All the mice are monitored for food intake, water consumption, and weight changes. Throughout the experiment, no Rb3/Rd-associated weight loss in mice is observed. In addition, none of the treated mice show variations in food and water consumption. Whereas, the number and size of the polyps are effectively reduced by Rb3 treatments[3]. |
| Kinase Assay | HepG2 cells are seeded at a concentration of 1×105 cells/mL (1.5 mL) in a 12-well plate and grown for 24 h. All cells are then transfected with Pnf-κB-luc plasmid (0.5 μg/well). Transfection are performed by the lipofectamine LTX. After 23 h of transfection, medium is changed to assay medium. After 24 h of transfection, cells are treated with test compounds (e.g., Ginsenoside Rb3; 0.1, 1 and 10 uM) for another 24 hours. After 25 h of transfection, cells are treated with 10 ng/mL of TNF-α for another 23 hours. The luciferase activity of cell lysates is assayed with 100 μL of by luciferase assay kit using an LB 953 Autolumat. Transfections are performed in triplicate, and activation is normalized against α-galactosidase activity[1]. |
| Cell Assay | NGF-differentiated PC12 cells are plated at a density of 1.0×105 cells/mL 2 days before each experiment. To initiate OGD, the cell culture medium is removed and replaced with the glucose-free DMEM, then the cells are incubated at 37°C in an oxygen-free chamber (95% N2 and 5% CO2) for 4 h (OGD), and the change in oxygen levels of the culture medium are monitored during incubation in oxygen-free chamber. Following OGD, glucose is added to normal levels (final concentration: 4.5 mg/mL) and cells are incubated under normal growth conditions (95% air and 5% CO2) for additional 24 h as OGD-reperfusion (OGD-Rep). Ginsenoside Rb3 (0.1, 1, 10 μM) is added to the culture 24 h before OGD treatment and throughout the OGD reperfusion. The control culture is always maintained in normal DMEM and put in the incubator under normal conditions[2]. |
| Animal Admin | Mice[3] Heterozygous male ApcMin/+ (C57BL/6J-ApcMin/+) mice are used. Total 32 male ApcMin/+ mice (aged 6 weeks) are divided into three groups; 10 mice in the control group and 22 mice equally divided for Rb3 and Rd treatments. The mice are daily gavage with a single dose of Ginsenoside Rb3 or Rd at 20 mg/kg, or solvent control. The treatments are carried out for 8 consecutive weeks. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 1117.1±65.0 °C at 760 mmHg |
| Molecular Formula | C53H90O22 |
| Molecular Weight | 1079.269 |
| Flash Point | 629.4±34.3 °C |
| Exact Mass | 1078.592407 |
| PSA | 357.06000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.622 |
| InChIKey | NODILNFGTFIURN-UHFFFAOYSA-N |
| SMILES | CC(C)=CCCC(C)(OC1OC(COC2OCC(O)C(O)C2O)C(O)C(O)C1O)C1CCC2(C)C1C(O)CC1C3(C)CCC(OC4OC(CO)C(O)C(O)C4OC4OC(CO)C(O)C(O)C4O)C(C)(C)C3CCC12C |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
[A quantitative method using one marker for simultaneous assay of ginsenosides in Panax ginseng and P. notoginseng].
Yao Xue Xue Bao 43(12) , 1211-6, (2008) Current quality control patterns are limited to industrial application, for most of the natural chemical reference substances are expensive and unavailable. Herein, a method, quantitative analysis of ... |
|
|
Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3.
Am. J. Chin. Med. 37(4) , 759-70, (2009) In order to investigate the mechanisms underlying the neuroprotective effect of ginsenoside Rb3, rat hippocampal neurons were primarily cultured, and exposed to 1 mM N-methyl-D-aspartate (NMDA), cell ... |
|
|
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats.
Pharm. Biol. 49(9) , 900-6, (2011) Panax ginseng C. A. Mey (Araliaceae) has been widely used in clinic for treatment of cardiovascular diseases in China. Ginsenoside Rb3 is the main chemical component of Panax ginseng.The aim of this s... |
| 20(S)-ginsenoside Rg3 |
| 20-((6-O-α-L-Arabinopyranosyl-β-D-glucopyranosyl)oxy)-12β-hydroxydammar-24-en-3β-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside |
| (3β,12β)-20-{[2-O-(β-D-Glucopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl 6-O-β-D-xylopyranosyl-α-D-glucopyranoside |
| Ginsenoside Rg3 (R-) |
| ginsenoside-RB2 |
| 20(R)Ginsenoside Rg3 |
| (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(10R,12S,13R,14R,17S)-12-hydroxy-17-[(2R)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,10,13,14-pentamethyl-2,3,5,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol |
| GinsenosideRb3 |
| (2S,3R,4S,5S,6R)-2-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxyméthyl)-2-({(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentaméthyl-17-[(2S)-6-méthyl-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5S)-3,4,5-trihydroxytétrahydro-2H-pyran-2-yl]oxy}méthyl)tétrahydro-2H-pyran-2-yl]oxy}-5-heptèn-2-yl]hexadécahydro-1H-cyclopenta[a]phénanthrén-3-yl}oxy)tétrahydro-2H-pyran-3-yl]oxy}-6-(hydroxyméthyl)tétrahydro-2H-pyran-3,4,5-triol |
| 3-epicabraleahydroxylactone |
| (2S,3R,4S,5S,6R)-2-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-2-({(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5S)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl]oxy}methyl)tetrahydro-2H-pyran-2-yl]oxy}-5-hepten-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl}oxy)tetrahydro-2H-pyran-3-yl]oxy}-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| (3β,12β)-20-{[6-O-(α-L-Arabinopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl-2-O-β-D-glucopyranosyl-β-D-glucopyranoside |
| 3|A-Acetoxy-22,23-dinorchol-5-en-24-al |
| (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(10R,12S,13R,14R,17S)-12-hydroxy-17-[(1R)-1-hydroxy-1,5-dimethyl-hex-4-enyl]-4,4,10,13,14-pentamethyl-2,3,5,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-methylol-tetrahydropyran-3-yl]oxy-6-methylol-tetrahydropyran-3,4,5-triol |
| Ginsenoside Rb3 |
| α-D-Glucopyranoside, (3β,12β)-20-[(2-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 6-O-β-D-xylopyranosyl- |
| (3|A,20S)-20-Formyl-3-hydroxy-5-pregnene 3-O-AcetateDiscontinued |
| (3β,12β)-20-{[6-O-(α-L-Arabinopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside |
| Ginsenoside Rg3 Rh2 |
| β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinopyranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl- |
| (20S)-3|A-Acetoxypregn-5-ene-20-carboxaldehyde |
| (3|A,20S)-3-(Acetyloxy)pregn-5-ene-20-carboxaldehyde |
| R-form-Ginsenoside Rg3 |
| GINSENOSIDE RG3(R-FORM)(P) |
| 3|A-Acetoxybisnor-5-cholen-22-al |

