Boc-L-aspartic acid 4-benzyl ester

Boc-L-aspartic acid 4-benzyl ester structure
|
Common Name | Boc-L-aspartic acid 4-benzyl ester | ||
|---|---|---|---|---|
| CAS Number | 7536-58-5 | Molecular Weight | 323.341 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 508.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H21NO6 | Melting Point | 98-102ºC | |
| MSDS | USA | Flash Point | 261.1±30.1 °C | |
Use of Boc-L-aspartic acid 4-benzyl ester(2S)-4-(Benzyloxy)-2-[(tert-butoxycarbonyl)amino]-4-oxobutanoic acid is an aspartic acid derivative[1]. |
| Name | Boc-L-Asp(OBzl)-OH |
|---|---|
| Synonym | More Synonyms |
| Description | (2S)-4-(Benzyloxy)-2-[(tert-butoxycarbonyl)amino]-4-oxobutanoic acid is an aspartic acid derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 508.1±50.0 °C at 760 mmHg |
| Melting Point | 98-102ºC |
| Molecular Formula | C16H21NO6 |
| Molecular Weight | 323.341 |
| Flash Point | 261.1±30.1 °C |
| Exact Mass | 323.136902 |
| PSA | 101.93000 |
| LogP | 3.24 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.527 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Vitamin K1 dependent carboxylase: beta-carboxylation of t-butyloxycarbonylaspartic acid alpha-benzyl ester.
Biochem. Biophys. Res. Commun. 107(1) , 246-9, (1982)
|
|
|
Vitamin K-dependent carboxylase. Carboxylation of aspartyl residues to beta-carboxyaspartyl residues in synthetic substrates.
J. Biol. Chem. 259(7) , 4272-8, (1984) The rat liver microsomal vitamin K-dependent carboxylase has been shown to carboxylate the aspartyl residue in N alpha-t-butyloxycarbonylaspartic acid alpha-benzyl ester to a beta-carboxyaspartyl resi... |
|
|
Transesterification and amide cis-trans isomerization in Zn and Cd complexes of the chelating amino acid ligand Boc-Asp(Dpa)-OBzl.
Dalton Trans. (1) , 154-62, (2007) The amino acid derivative Boc-Asp-OBzl (Boc=N-butyloxycarbonyl; Asp=aspartic acid; Bzl=benzyl) was functionalized by coupling its carboxylate side chain to dipicolylamine. This yielded the tridentate ... |
| (2S)-4-(benzyloxy)-2-[(tert-butoxycarbonyl)amino]-4-oxobutanoic acid (non-preferred name) |
| EINECS 231-406-8 |
| N-tert-Butoxycarbonyl-L-aspartic acid 4-benzyl ester |
| MFCD00065564 |
| (2S)-4-(Benzyloxy)-2-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)-4-oxobutanoic acid |
| (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxo-4-phenylmethoxybutanoic acid |
| L-Aspartic acid, N-((1,1-dimethylethoxy)carbonyl)-, 4-(phenylmethyl) ester |
| L-Aspartic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 4-(phenylmethyl) ester |
| N-α-t-BOC-L-aspartic-β-benzyl ester |
| Boc-Asp(OBzl)-OH |
| Boc-L-asparticacid4-benzylester |
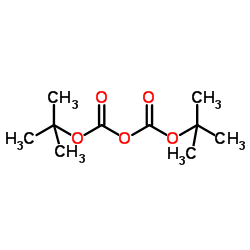 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:2177-63-1
CAS#:2177-63-1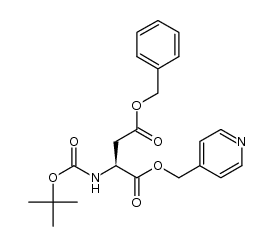 CAS#:1361400-95-4
CAS#:1361400-95-4 CAS#:2886-33-1
CAS#:2886-33-1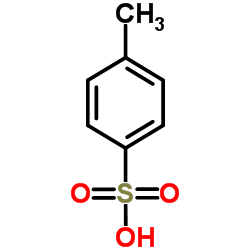 CAS#:104-15-4
CAS#:104-15-4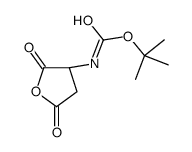 CAS#:30750-74-4
CAS#:30750-74-4 CAS#:100-51-6
CAS#:100-51-6 CAS#:56-84-8
CAS#:56-84-8 CAS#:34582-32-6
CAS#:34582-32-6 CAS#:23446-11-9
CAS#:23446-11-9![(3S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-4-hydroxybutanoic acid structure](https://image.chemsrc.com/caspic/233/83345-44-2.png) CAS#:83345-44-2
CAS#:83345-44-2![2-Methyl-2-propanyl N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-homoserinate structure](https://image.chemsrc.com/caspic/130/81323-58-2.png) CAS#:81323-58-2
CAS#:81323-58-2 CAS#:26048-69-1
CAS#:26048-69-1 CAS#:65710-57-8
CAS#:65710-57-8 CAS#:59768-74-0
CAS#:59768-74-0 CAS#:6327-59-9
CAS#:6327-59-9 CAS#:104227-71-6
CAS#:104227-71-6
