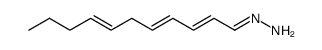
Triacsin C from Streptomyces sp.
Triacsin C from Streptomyces sp. structure
|
Common Name | Triacsin C from Streptomyces sp. | ||
|---|---|---|---|---|
| CAS Number | 76896-80-5 | Molecular Weight | 207.272 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 320.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H17N3O | Melting Point | 100-102ºC | |
| MSDS | USA | Flash Point | 147.7±28.7 °C | |
Use of Triacsin C from Streptomyces sp.Triacsin C (WS 1228A), a natural intracellular long-chain acyl-CoA synthetases (ACSL) inhibitor, is from Streptomyces aureofaciens. Triacsin C inhibits TAG accumulation into lipid droplets (LD) by suppressing ACSL activity[1]. Triacsin C is found to be highly effective against rotavirus replication[2]. |
| Name | triacsin c |
|---|---|
| Synonym | More Synonyms |
| Description | Triacsin C (WS 1228A), a natural intracellular long-chain acyl-CoA synthetases (ACSL) inhibitor, is from Streptomyces aureofaciens. Triacsin C inhibits TAG accumulation into lipid droplets (LD) by suppressing ACSL activity[1]. Triacsin C is found to be highly effective against rotavirus replication[2]. |
|---|---|
| Related Catalog | |
| Target |
ACSL[1] |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 320.7±45.0 °C at 760 mmHg |
| Melting Point | 100-102ºC |
| Molecular Formula | C11H17N3O |
| Molecular Weight | 207.272 |
| Flash Point | 147.7±28.7 °C |
| Exact Mass | 207.137161 |
| PSA | 53.82000 |
| LogP | 4.02 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.485 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 3 |
|
~% 
Triacsin C from... CAS#:76896-80-5 |
| Literature: Tanaka; Yoshida; Itoh; Imanaka Tetrahedron Letters, 1981 , vol. 22, # 35 p. 3421 - 3422 |
|
~% 
Triacsin C from... CAS#:76896-80-5 |
| Literature: Tanaka; Yoshida; Itoh; Imanaka Tetrahedron Letters, 1981 , vol. 22, # 35 p. 3421 - 3422 |
|
Acyl-CoA synthetase as a cancer survival factor: its inhibition enhances the efficacy of etoposide.
Cancer Sci. 100(8) , 1556-62, (2009) Lipid metabolism is often elevated in cancer cells and plays an important role in their growth and malignancy. Acyl-CoA synthetase (ACS), which converts long-chain fatty acids to acyl-CoA, is overexpr... |
|
|
Human intestinal acyl-CoA synthetase 5 is sensitive to the inhibitor triacsin C.
World J. Gastroenterol. 17(44) , 4883-9, (2011) To investigate whether human acyl-CoA synthetase 5 (ACSL5) is sensitive to the ACSL inhibitor triacsin C.The ACSL isoforms ACSL1 and ACSL5 from rat as well as human ACSL5 were cloned and recombinantly... |
|
|
Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization.
J. Thorac. Cardiovasc. Surg. 284 , 30941-8, (2009) Fatty acid-induced triacylglycerol synthesis produces triacylglycerol droplets with a protein coat that includes perilipin 3/TIP47 and perilipin 4/S3-12. This study addresses the following two questio... |
| (3E)-1-Oxo-3-[(2E,4E,7E)-2,4,7-undecatrien-1-ylidene]triazane |
| 2E,4E,7E-undecatriene-1-triazine |
| Antibiotic WS-1228A |
| WS 1228A |
| Triacsincapprox. |
| triacsin c from streptomyces sp. |
| Triacsin C |
| 2,4,7-Undecatrienal |
| WS1228A |
| Galloflavin |
| 2E,4E,7E-UNDECATRIENE-1-TRIAZENE |
| 2,4,7-Undecatrienal, 2-nitrosohydrazone, (1E,2E,4E,7E)- |
| 1-hydroxy-3-(E,E,E,-2',4',7'-undecatrienylidine)triazene |