Roxane
Modify Date: 2024-01-09 15:59:33
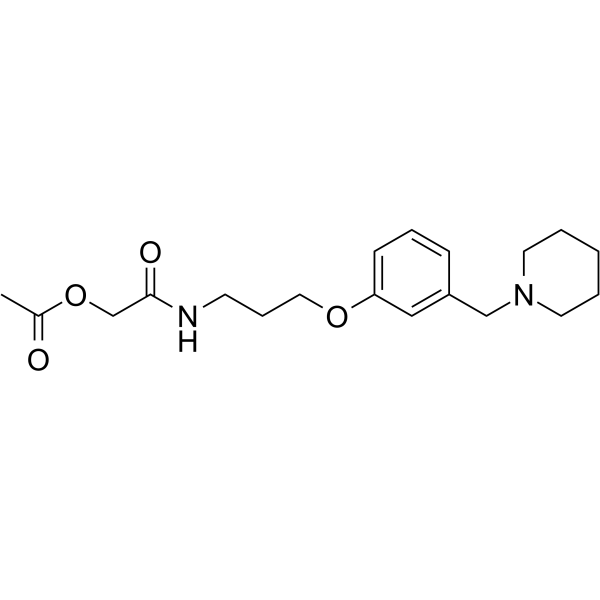
Roxane structure
|
Common Name | Roxane | ||
|---|---|---|---|---|
| CAS Number | 78628-28-1 | Molecular Weight | 348.437 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 537.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H28N2O4 | Melting Point | 225 °C (dec.)(lit.) | |
| MSDS | N/A | Flash Point | 278.7±28.7 °C | |
Use of RoxaneRoxatidine acetate is a potent, selective, competitive and orally active histamine H2-receptor antagonist. Roxatidine acetate has antisecretory potency against gastric acid secretion. Roxatidine acetate can also suppress inflammatory responses and can be used for gastric and duodenal ulcers research. Roxatidine acetate has antitumor activity[1][2][3]. |
| Name | [2-oxo-2-[3-[3-(piperidin-1-ylmethyl)phenoxy]propylamino]ethyl] acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Roxatidine acetate is a potent, selective, competitive and orally active histamine H2-receptor antagonist. Roxatidine acetate has antisecretory potency against gastric acid secretion. Roxatidine acetate can also suppress inflammatory responses and can be used for gastric and duodenal ulcers research. Roxatidine acetate has antitumor activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
H2 Receptor |
| In Vitro | Roxatidine acetate (0-120 μM, 1 h) suppresses inflammatory responses via inhibition of NF-κB and p38 MAPK activation in LPS-induced RAW 264.7 macrophages[2]. Roxatidine acetate (6.25 μM, 12.5 μM, and 25 μM; pre-treatment for 30 min) suppresses the PMACI-induced activation of p38 MAPK, but does not affect the phosphorylation of ERK or JNK. The total ERK 1/2, JNK, and p38 MAPK levels are unaffected by roxatidine in human mast-cells-1 (HMC-1) cells[4]. Western Blot Analysis[2] Cell Line: RAW 264.7 Concentration: 40, 80, and 120 μM Incubation Time: 1 h Result: Suppressed LPS-induced PGE2, NO, and histamine production and COX-2, iNOS, and HDC expressions. Inhibited expressions of TNF-α, IL-1β, IL-6, and VEGF-1. Concentration-dependently attenuated the nuclear translocations of p65 and p50. Inhibited LPS-induced phosphorylation of p38 MAP kinase. Significantly down-regulated the LPS-induced productions of NO and PGE2 (prostaglandin E2). |
| In Vivo | Roxatidine acetate (0-300 mg/kg; p.o.; 26 days) suppressed growth of Colon 38 tumor implants in mice[3]. Roxatidine acetate (oral gavage; 20 mg/kg; single dose) inhibits Compound 48/80-increased TNF-α, IL-6, and IL-1β production and mRNA expression. Additionally, Roxatidine decreases the compound 48/80-induced degradation of procaspase-1 and appearance of the corresponding cleaved bands in mice[4]. Animal Model: Male C57BL/6 Colon 38-bearing mice (8-week-old, 20 – 22 g)[3] Dosage: 30, 100, and 300 mg/kg per day, 1 ml/100 g body weight Administration: Oral administration, 29 days beginning 3 days before Colon 38 implantation or 26 days beginning concomitantly with Colon 38 implantation Result: Suppressed growth of Colon 38 tumor implants in a dose-related manner after day 26. Suppressed VEGF levels in tumor tissue and significantly decreased serum VEGF levels. Animal Model: ICR male mice (6 weeks old)[4] Dosage: 20 mg/kg Administration: Oral gavage; 20 mg/kg; single dose Result: Suppressed compound 48/80-induced allergic inflammation in anaphylactic animal model. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 537.3±45.0 °C at 760 mmHg |
| Melting Point | 225 °C (dec.)(lit.) |
| Molecular Formula | C19H28N2O4 |
| Molecular Weight | 348.437 |
| Flash Point | 278.7±28.7 °C |
| Exact Mass | 348.204895 |
| PSA | 67.87000 |
| LogP | 2.00 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.533 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . S36:Wear suitable protective clothing . S37/39:Wear suitable gloves and eye/face protection . |
| WGK Germany | 3 |
| RTECS | HA1595000 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Roxatidine acetate |
| Roxatidinum [Latin] |
| Pifatidine |
| Xarcin |
| 2-Oxo-2-({3-[3-(1-piperidinylmethyl)phenoxy]propyl}amino)ethyl acetate |
| ACETAMIDE, 2-HYDROXY-N-(3-(m-(PIPERIDINOMETHYL)PHENOXY)PROPYL)-, ACETATE (ester) |
| Roxit |
| Gastralgin |
| Acetamide, 2-(acetyloxy)-N-[3-[3-(1-piperidinylmethyl)phenoxy]propyl]- |
| Roxatidina |
| Roxatidinum |
| 2-(Acetyloxy)-N-[3-[3-(1-piperidinylmethyl)phenoxy]propyl]acetamide |
| 2-Oxo-2-({3-[3-(piperidin-1-ylmethyl)phenoxy]propyl}amino)ethylacetat |
| 2-(Acetyloxy)-N-(3-(3-(1-piperidinylmethyl)phenoxy)propyl)acetamide |
| Altat |
| Roxatidina [Spanish] |
| MFCD00866898 |
| Roxane |
| 2-Oxo-2-({3-[3-(piperidin-1-ylmethyl)phenoxy]propyl}amino)ethyl acetate |
| Aceroxatidine |