4-Phenoxyphenol
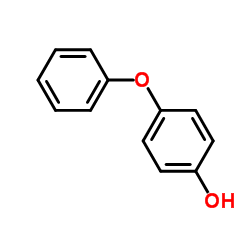
4-Phenoxyphenol structure
|
Common Name | 4-Phenoxyphenol | ||
|---|---|---|---|---|
| CAS Number | 831-82-3 | Molecular Weight | 186.207 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 313.8±25.0 °C at 760 mmHg | |
| Molecular Formula | C12H10O2 | Melting Point | 80-84 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 141.4±17.4 °C | |
Use of 4-PhenoxyphenolBIF-44, also known as 4-Phenoxyphenol and Hydroquinone monophenyl ether, is a sensitizer of BCL-2-associated X protein (BAX) activation by binding to a pocket formed by the junction of the α3-α4 and α5-α6 hairpins. BIF-44 enhances BAX activity. |
| Name | 4-phenoxyphenol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 313.8±25.0 °C at 760 mmHg |
| Melting Point | 80-84 °C(lit.) |
| Molecular Formula | C12H10O2 |
| Molecular Weight | 186.207 |
| Flash Point | 141.4±17.4 °C |
| Exact Mass | 186.068085 |
| PSA | 29.46000 |
| LogP | 3.35 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.605 |
| InChIKey | ZSBDGXGICLIJGD-UHFFFAOYSA-N |
| SMILES | Oc1ccc(Oc2ccccc2)cc1 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | insoluble |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Packaging Group | I; II; III |
| HS Code | 29095090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2909500000 |
|---|---|
| Summary | 2909500000 ether-phenols, ether-alcohol-phenols and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspas... |
|
|
Activation and inhibition of leukotriene A4 hydrolase aminopeptidase activity by diphenyl ether and derivatives.
Bioorg. Med. Chem. Lett. 18 , 6549-52, (2008) The synthesis and biological evaluation of a series of diphenyl ether derivatives were described. The compounds can either activate or inhibit the aminopeptidase activity of leukotriene A(4) hydrolase... |
| 4-Phenoxyphenol |
| o-Phenylhydroquinone |
| EINECS 212-611-1 |
| MFCD00002331 |
| Phenol, 4-phenoxy- |
 CAS#:3379-38-2
CAS#:3379-38-2 CAS#:1655-69-2
CAS#:1655-69-2 CAS#:5031-78-7
CAS#:5031-78-7 CAS#:7005-72-3
CAS#:7005-72-3 CAS#:19082-52-1
CAS#:19082-52-1 CAS#:462-06-6
CAS#:462-06-6 CAS#:123-31-9
CAS#:123-31-9 CAS#:108-90-7
CAS#:108-90-7 CAS#:108-86-1
CAS#:108-86-1 CAS#:106-41-2
CAS#:106-41-2![[4-(4-phenoxyphenoxy)phenyl]-phenylmethanone structure](https://image.chemsrc.com/caspic/484/106315-29-1.png) CAS#:106315-29-1
CAS#:106315-29-1 CAS#:557-40-4
CAS#:557-40-4 CAS#:25345-76-0
CAS#:25345-76-0 CAS#:38778-05-1
CAS#:38778-05-1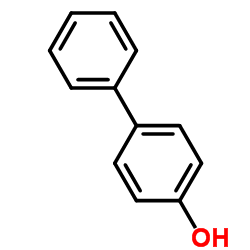 CAS#:92-69-3
CAS#:92-69-3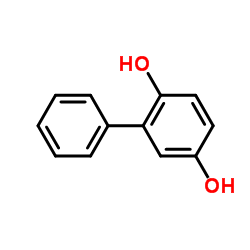 CAS#:1079-21-6
CAS#:1079-21-6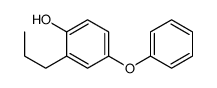 CAS#:194792-58-0
CAS#:194792-58-0 CAS#:71-43-2
CAS#:71-43-2
