(S)-ropivacaine
Modify Date: 2025-08-24 20:43:30
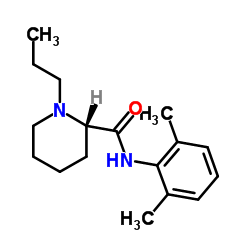
(S)-ropivacaine structure
|
Common Name | (S)-ropivacaine | ||
|---|---|---|---|---|
| CAS Number | 854056-07-8 | Molecular Weight | 274.401 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 410.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C18H30N2O4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 201.9±28.7 °C | |
Use of (S)-ropivacaineRopivacaine mesylate is an aminoamide compound, effectively blocks neuropathic pain. Ropivacaine mesylate is an inhibitor of K2P (two-pore domain potassium channel) TREK-1 with an IC50 of 402.7 μM in COS-7 cell's membrane. Ropivacaine mesylate inhibits pressure-induces lung endothelial hyperpermeability in models of acute hypertension[1][2]. |
| Name | Ropivacaine mesylate |
|---|---|
| Synonym | More Synonyms |
| Description | Ropivacaine mesylate is an aminoamide compound, effectively blocks neuropathic pain. Ropivacaine mesylate is an inhibitor of K2P (two-pore domain potassium channel) TREK-1 with an IC50 of 402.7 μM in COS-7 cell's membrane. Ropivacaine mesylate inhibits pressure-induces lung endothelial hyperpermeability in models of acute hypertension[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Epidural administration of Ropivacaine mesylate effectively blocks neuropathic pain (both mechanical allodynia and heat hyperalgesia) without induction of analgesic tolerance and significantly delays the development of neuropathic pain produced by peripheral nerve injury[1]. Ropivacaine mesylate inhibits pressure-induced increases in filtration coefficient (Kf) without affecting pulmonary artery pressure (Ppa), pulmonary capillary pressures (Ppc), and zonal characteristics (ZC)[2]. Ropivacaine mesylate prevents pressure-induced lung edema and associated hyperpermeability as evidence by maintaining PaO2, lung wet-to-dry ratio and plasma volume in levels similar to sham rats[2]. Ropivacaine mesylate inhibits pressure-induced NO production as evidenced by decreased lung nitro-tyrosine content when compared to hypertensive lungs[2]. Animal Model: Adult Sprague-Dawley rats (300–400g)[2] Dosage: 1 μM Administration: Infusion (added to the perfusate reservoir) Result: Attenuated pressure-dependent increases in filtration coefficient (Kf). |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 410.2±45.0 °C at 760 mmHg |
| Molecular Formula | C18H30N2O4S |
| Molecular Weight | 274.401 |
| Flash Point | 201.9±28.7 °C |
| Exact Mass | 274.204498 |
| PSA | 95.09000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.552 |
| HS Code | 2942000000 |
|---|
| HS Code | 2942000000 |
|---|
| (2S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide |
| (S)-(-)-1-propyl-2',6'-pipecoloxylidide |
| l-N-n-Propylpipecolic acid-2,6-xylidide |
| (-)-1-Propyl-2',6'-pipecoloxylidide |
| UNII-7IO5LYA57N |
| (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilide |
| 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2S)- |
| Ropivacaine |
| Ropivacaine Mesylate API |
| Ropivacainemesylate |
| (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide |
| (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide |

