Benazepril hydrochloride
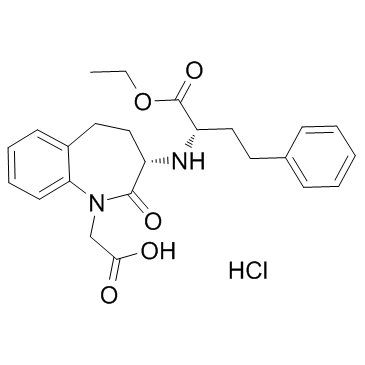
Benazepril hydrochloride structure
|
Common Name | Benazepril hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 86541-74-4 | Molecular Weight | 460.951 | |
| Density | N/A | Boiling Point | 691.2ºC at 760 mmHg | |
| Molecular Formula | C24H29ClN2O5 | Melting Point | 188-190°C | |
| MSDS | USA | Flash Point | 371.8ºC | |
Use of Benazepril hydrochlorideBenazepril hydrochloride, an angiotensin converting enzyme inhibitor, which is a medication used to treat high blood pressure.Target: angiotensin converting enzyme (ACE)Benazepril hydrochloride is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril hydrochloride is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor [1].Animals were randomly divided into 4 groups: sham STNx group (control), STNx group, morning benazepril hydrochloride group (MB) and evening benazepril hydrochloride group (EB).Benazepril hydrochloride was intragastrically administered at a dose of 10 mg/kg/day at 07:00 and 19:00 in the MB group and EB group respectively for 12 weeks. All the animals were synchronized to the light:dark cycle of 12:12 for 12 weeks. Systolic blood pressure (SBP), 24-h urinary protein excretion and renal function were measured at 11 weeks. Blood samples and kidneys were collected every 4 h throughout a day to detect the expression pattern of renin activity (RA), angiotensin II (AngII) and aldosterone (Ald) by radioimmunoassay (RIA) and the mRNA expression profile of clock genes (bmal1, dbp and per2) by real-time PCR at 12 weeks. Our results showed that no significant differences were noted in the SBP, 24-h urine protein excretion and renal function between the MB and EB groups. There were no significant differences in average Ald and RA content of a day between the MB group and EB group. The expression peak of bmal1 mRNA was phase-delayed by 4 to 8 h, and the diurnal variation of per2 and dbp mRNA diminished in the MB and EB groups compared with the control and STNx groups. It was concluded when the similar SBP reduction, RAAS inhibition and clock gene profile were achieved with optimal dose of benazepril hydrochloride, morning versus evening dosing of benazepril hydrochloride has the same renoprotection effects [2].Clinical indications: Congestive heart failure; End stage renal disease; HypertensionFDA Approved Date: Toxicity: headaches; cough; Anaphylaxis; angioedema; hyperkalemia |
| Name | Benazepril Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Benazepril hydrochloride, an angiotensin converting enzyme inhibitor, which is a medication used to treat high blood pressure.Target: angiotensin converting enzyme (ACE)Benazepril hydrochloride is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril hydrochloride is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor [1].Animals were randomly divided into 4 groups: sham STNx group (control), STNx group, morning benazepril hydrochloride group (MB) and evening benazepril hydrochloride group (EB).Benazepril hydrochloride was intragastrically administered at a dose of 10 mg/kg/day at 07:00 and 19:00 in the MB group and EB group respectively for 12 weeks. All the animals were synchronized to the light:dark cycle of 12:12 for 12 weeks. Systolic blood pressure (SBP), 24-h urinary protein excretion and renal function were measured at 11 weeks. Blood samples and kidneys were collected every 4 h throughout a day to detect the expression pattern of renin activity (RA), angiotensin II (AngII) and aldosterone (Ald) by radioimmunoassay (RIA) and the mRNA expression profile of clock genes (bmal1, dbp and per2) by real-time PCR at 12 weeks. Our results showed that no significant differences were noted in the SBP, 24-h urine protein excretion and renal function between the MB and EB groups. There were no significant differences in average Ald and RA content of a day between the MB group and EB group. The expression peak of bmal1 mRNA was phase-delayed by 4 to 8 h, and the diurnal variation of per2 and dbp mRNA diminished in the MB and EB groups compared with the control and STNx groups. It was concluded when the similar SBP reduction, RAAS inhibition and clock gene profile were achieved with optimal dose of benazepril hydrochloride, morning versus evening dosing of benazepril hydrochloride has the same renoprotection effects [2].Clinical indications: Congestive heart failure; End stage renal disease; HypertensionFDA Approved Date: Toxicity: headaches; cough; Anaphylaxis; angioedema; hyperkalemia |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 691.2ºC at 760 mmHg |
|---|---|
| Melting Point | 188-190°C |
| Molecular Formula | C24H29ClN2O5 |
| Molecular Weight | 460.951 |
| Flash Point | 371.8ºC |
| Exact Mass | 460.176514 |
| PSA | 95.94000 |
| LogP | 3.83100 |
| InChIKey | VPSRQEHTHIMDQM-FKLPMGAJSA-N |
| SMILES | CCOC(=O)C(CCc1ccccc1)NC1CCc2ccccc2N(CC(=O)O)C1=O.Cl |
| Storage condition | Desiccate at +4°C |
| Water Solubility | DMSO: ~34 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | CX7065000 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: implications for in vitro-in vivo extrapolation of clearance of prodrugs.
Drug Metab. Dispos. 42(9) , 1522-31, (2014) Hydrolysis plays an important role in metabolic activation of prodrugs. In the current study, species and in vitro system differences in hepatic and extrahepatic hydrolysis were investigated for 11 pr... |
|
|
Trough:peak ratio of the blood pressure response to angiotensin converting enzyme inhibitors.
Med. Dosim. 12 , S91-S94, (1994) SHORT- VERSUS LONG-ACTING ANGIOTENSIN CONVERTING ENZYME (ACE) INHIBITORS: Although ACE inhibitors are widely used in the treatment of hypertension, there are few data on trough:peak ratios and the dat... |
|
|
Using ACE inhibitors appropriately.
Am. Fam. Physician 66 , 461-468, (2002) When first introduced in 1981, angiotensin-converting enzyme (ACE) inhibitors were indicated only for treatment of refractory hypertension. Since then, they have been shown to reduce morbidity or mort... |
| [(3S)-3-{[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]essigsäurehydrochlorid |
| 1H-1-Benzazepine-1-acetic acid, 3-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-, (3S)-, hydrochloride (1:1) |
| [(3S)-3-{[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid hydrochloride |
| Benazepril Hcl |
| [(3S)-3-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid hydrochloride |
| 2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid,hydrochloride |
| acide [(3S)-3-{[(1S)-1-(éthoxycarbonyl)-3-phénylpropyl]amino}-2-oxo-2,3,4,5-tétrahydro-1H-1-benzazépin-1-yl]acétique chlorhydrate |
| [(3S)-3-{[(2S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid hydrochloride (1:1) |
| (3S)-3-(((1S)-1-Carboxy-3-phenylpropyl)amino)-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic Acid 3-Ethyl Ester Monohydrochloride |
| [(3S)-3-{[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid hydrochloride (1:1) |
| [(3S)-3-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid hydrochloride |
| MFCD01668249 |
| 1H-1-benzazepine-1-acetic acid, 3-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-, (3S)-, monohydrochloride |
![(2S,3'S)-2-(1-tert-butoxycarbonylmethyl-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-ylamino)-4-phenylbutyric acid ethyl ester Structure](https://image.chemsrc.com/caspic/207/109010-61-9.png) CAS#:109010-61-9
CAS#:109010-61-9 CAS#:859635-53-3
CAS#:859635-53-3 CAS#:86541-75-5
CAS#:86541-75-5 CAS#:64920-29-2
CAS#:64920-29-2 CAS#:86499-53-8
CAS#:86499-53-8![4,5-Dihydro-1H-benzo[b]azepin-2(3H)-one Structure](https://image.chemsrc.com/caspic/098/4424-80-0.png) CAS#:4424-80-0
CAS#:4424-80-0 CAS#:86499-22-1
CAS#:86499-22-1 CAS#:86499-23-2
CAS#:86499-23-2