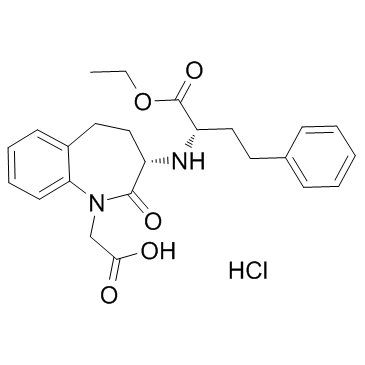
Benazepril
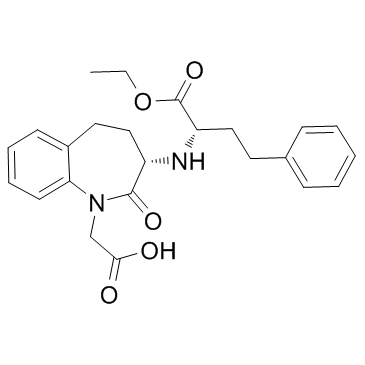
Benazepril structure
|
Common Name | Benazepril | ||
|---|---|---|---|---|
| CAS Number | 86541-75-5 | Molecular Weight | 424.490 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 691.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C24H28N2O5 | Melting Point | 133-135 °C(lit.) | |
| MSDS | N/A | Flash Point | 371.8±31.5 °C | |
Use of BenazeprilBenazepril, an angiotensin converting enzyme inhibitor, which is a medication used to treat high blood pressure.Target: angiotensin converting enzyme (ACE)Benazepril is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor [1].Animals were randomly divided into 4 groups: sham STNx group (control), STNx group, morning benazepril group (MB) and evening benazepril group (EB).Benazepril was intragastrically administered at a dose of 10 mg/kg/day at 07:00 and 19:00 in the MB group and EB group respectively for 12 weeks. All the animals were synchronized to the light:dark cycle of 12:12 for 12 weeks. Systolic blood pressure (SBP), 24-h urinary protein excretion and renal function were measured at 11 weeks. Blood samples and kidneys were collected every 4 h throughout a day to detect the expression pattern of renin activity (RA), angiotensin II (AngII) and aldosterone (Ald) by radioimmunoassay (RIA) and the mRNA expression profile of clock genes (bmal1, dbp and per2) by real-time PCR at 12 weeks. Our results showed that no significant differences were noted in the SBP, 24-h urine protein excretion and renal function between the MB and EB groups. There were no significant differences in average Ald and RA content of a day between the MB group and EB group. The expression peak of bmal1 mRNA was phase-delayed by 4 to 8 h, and the diurnal variation of per2 and dbp mRNA diminished in the MB and EB groups compared with the control and STNx groups. It was concluded when the similar SBP reduction, RAAS inhibition and clock gene profile were achieved with optimal dose of benazepril, morning versus evening dosing of benazepril has the same renoprotection effects [2].Clinical indications: Congestive heart failure; End stage renal disease; HypertensionFDA Approved Date: Toxicity: headaches; cough; Anaphylaxis; angioedema; hyperkalemia |
| Name | benazepril |
|---|---|
| Synonym | More Synonyms |
| Description | Benazepril, an angiotensin converting enzyme inhibitor, which is a medication used to treat high blood pressure.Target: angiotensin converting enzyme (ACE)Benazepril is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor [1].Animals were randomly divided into 4 groups: sham STNx group (control), STNx group, morning benazepril group (MB) and evening benazepril group (EB).Benazepril was intragastrically administered at a dose of 10 mg/kg/day at 07:00 and 19:00 in the MB group and EB group respectively for 12 weeks. All the animals were synchronized to the light:dark cycle of 12:12 for 12 weeks. Systolic blood pressure (SBP), 24-h urinary protein excretion and renal function were measured at 11 weeks. Blood samples and kidneys were collected every 4 h throughout a day to detect the expression pattern of renin activity (RA), angiotensin II (AngII) and aldosterone (Ald) by radioimmunoassay (RIA) and the mRNA expression profile of clock genes (bmal1, dbp and per2) by real-time PCR at 12 weeks. Our results showed that no significant differences were noted in the SBP, 24-h urine protein excretion and renal function between the MB and EB groups. There were no significant differences in average Ald and RA content of a day between the MB group and EB group. The expression peak of bmal1 mRNA was phase-delayed by 4 to 8 h, and the diurnal variation of per2 and dbp mRNA diminished in the MB and EB groups compared with the control and STNx groups. It was concluded when the similar SBP reduction, RAAS inhibition and clock gene profile were achieved with optimal dose of benazepril, morning versus evening dosing of benazepril has the same renoprotection effects [2].Clinical indications: Congestive heart failure; End stage renal disease; HypertensionFDA Approved Date: Toxicity: headaches; cough; Anaphylaxis; angioedema; hyperkalemia |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 691.2±55.0 °C at 760 mmHg |
| Melting Point | 133-135 °C(lit.) |
| Molecular Formula | C24H28N2O5 |
| Molecular Weight | 424.490 |
| Flash Point | 371.8±31.5 °C |
| Exact Mass | 424.199829 |
| PSA | 95.94000 |
| LogP | 3.86 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.608 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xn |
|---|---|
| Safety Phrases | S22-S24/25 |
|
~% 
Benazepril CAS#:86541-75-5 |
| Literature: WO2006/84761 A1, ; Page/Page column 22; 23 ; |
|
~% 
Benazepril CAS#:86541-75-5 |
| Literature: US4575503 A1, ; US4410520 A1, ; |
|
~% 
Benazepril CAS#:86541-75-5 |
| Literature: US4575503 A1, ; US4410520 A1, ; US4473575 A1, ; |
| Benazeprilum |
| [(3S)-3-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid |
| 2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid |
| acide [(3S)-3-{[(1S)-1-(éthoxycarbonyl)-3-phénylpropyl]amino}-2-oxo-2,3,4,5-tétrahydro-1H-1-benzazépin-1-yl]acétique |
| Benazeprilum [Latin] |
| benzazepril |
| 1H-1-Benzazepine-1-acetic acid, 3-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-, (3S)- |
| Benazepril [INN:BAN] |
| [S-(R*,R*)]-3-[[1-(Ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic Acid |
| benazapril |
| Benazepril |
| [(3S)-3-{[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid |
| (3S)-1-(carboxymethyl)-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-1H-[1]benzazepin-2-one |
| MFCD00864466 |
| [(3S)-3-({(1S)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}amino)-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid |