PAP-1
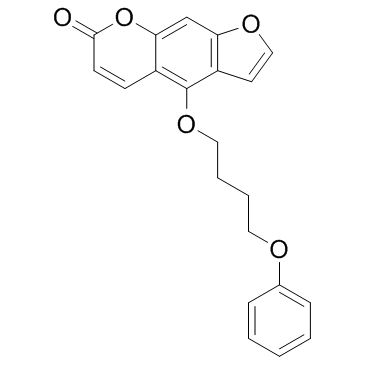
PAP-1 structure
|
Common Name | PAP-1 | ||
|---|---|---|---|---|
| CAS Number | 870653-45-5 | Molecular Weight | 350.365 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 555.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H18O5 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 289.9±30.1 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
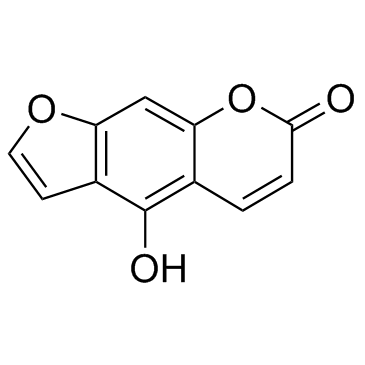
Use of PAP-1PAP-1 is a selective inhibitor of Kv1.3, voltage-gated K+ channel. PAP-1 (EC50=2 nM) potently inhibits human T effector memory cell proliferation and delayed hypersensitivity. IC50 value: 2 nM (EC50) [1]in vitro: blocks Kv1.3 in a use-dependent manner, with a Hill coefficient of 2 and an EC50 of 2 nM, by preferentially binding to the C-type inactivated state of the channel. PAP-1 is 23-fold selective over Kv1.5, 33- to 125-fold selective over other Kv1-family channels, and 500- to 7500-fold selective over Kv2.1, Kv3.1, Kv3.2, Kv4.2, HERG, calcium-activated K+ channels, Na+,Ca2+, and Cl- channels [1]. The blockade of Kv1.3 results in membrane depolarization and inhibition of TEM proliferation and function. In this study, the in vitro effects of PAP-1 on T cells and the in vivo toxicity and pharmacokinetics (PK) were examined in rhesus macaques (RM) with the ultimate aim of utilizing PAP-1 to define the role of TEMs in RM infected with simian immunodeficiency virus (SIV). Electrophysiologic studies on T cells in RM revealed a Kv1.3 expression pattern similar to that in human T cells. Thus, PAP-1 effectively suppressed TEM proliferation in RM [2].in vivo: PAP-1 does not exhibit cytotoxic or phototoxic effects, is negative in the Ames test, and affects cytochrome P450-dependent enzymes only at micromolar concentrations. PAP-1 potently inhibits the proliferation of human TEM cells and suppresses delayed type hypersensitivity, a TEM cell-mediated reaction, in rats [1]. When administered intravenously, PAP-1 showed a half-life of 6.4 hrs; the volume of distribution suggested extensive distribution into extravascular compartments. When orally administered, PAP-1 was efficiently absorbed. Plasma concentrations in RM undergoing a 30-day, chronic dosing study indicated that PAP-1 levels suppressive to TEMs in vitro can be achieved and maintained in vivo at a non-toxic dose [2]. |
| Name | 5-(4-Phenoxybutoxy)psoralen |
|---|---|
| Synonym | More Synonyms |
| Description | PAP-1 is a selective inhibitor of Kv1.3, voltage-gated K+ channel. PAP-1 (EC50=2 nM) potently inhibits human T effector memory cell proliferation and delayed hypersensitivity. IC50 value: 2 nM (EC50) [1]in vitro: blocks Kv1.3 in a use-dependent manner, with a Hill coefficient of 2 and an EC50 of 2 nM, by preferentially binding to the C-type inactivated state of the channel. PAP-1 is 23-fold selective over Kv1.5, 33- to 125-fold selective over other Kv1-family channels, and 500- to 7500-fold selective over Kv2.1, Kv3.1, Kv3.2, Kv4.2, HERG, calcium-activated K+ channels, Na+,Ca2+, and Cl- channels [1]. The blockade of Kv1.3 results in membrane depolarization and inhibition of TEM proliferation and function. In this study, the in vitro effects of PAP-1 on T cells and the in vivo toxicity and pharmacokinetics (PK) were examined in rhesus macaques (RM) with the ultimate aim of utilizing PAP-1 to define the role of TEMs in RM infected with simian immunodeficiency virus (SIV). Electrophysiologic studies on T cells in RM revealed a Kv1.3 expression pattern similar to that in human T cells. Thus, PAP-1 effectively suppressed TEM proliferation in RM [2].in vivo: PAP-1 does not exhibit cytotoxic or phototoxic effects, is negative in the Ames test, and affects cytochrome P450-dependent enzymes only at micromolar concentrations. PAP-1 potently inhibits the proliferation of human TEM cells and suppresses delayed type hypersensitivity, a TEM cell-mediated reaction, in rats [1]. When administered intravenously, PAP-1 showed a half-life of 6.4 hrs; the volume of distribution suggested extensive distribution into extravascular compartments. When orally administered, PAP-1 was efficiently absorbed. Plasma concentrations in RM undergoing a 30-day, chronic dosing study indicated that PAP-1 levels suppressive to TEMs in vitro can be achieved and maintained in vivo at a non-toxic dose [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 555.8±50.0 °C at 760 mmHg |
| Molecular Formula | C21H18O5 |
| Molecular Weight | 350.365 |
| Flash Point | 289.9±30.1 °C |
| Exact Mass | 350.115417 |
| PSA | 61.81000 |
| LogP | 4.56 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Storage condition | 2~8°C |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H334 |
| Precautionary Statements | P261-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Risk Phrases | R42/43 |
| Safety Phrases | 22-36/37-45 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2932999099 |
|
~60% 
PAP-1 CAS#:870653-45-5 |
| Literature: Schmitz, Alexander; Sankaranarayanan, Ananthakrishnan; Azam, Philippe; Schmidt-Lassen, Kristina; Homerick, Daniel; Haensel, Wolfram; Wulff, Heike Molecular Pharmacology, 2005 , vol. 68, # 5 p. 1254 - 1270 |
|
~% 
PAP-1 CAS#:870653-45-5 |
| Literature: Molecular Pharmacology, , vol. 68, # 5 p. 1254 - 1270 |
|
~% 
PAP-1 CAS#:870653-45-5 |
| Literature: Molecular Pharmacology, , vol. 68, # 5 p. 1254 - 1270 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 7H-Furo[3,2-g][1]benzopyran-7-one, 4-(4-phenoxybutoxy)- |
| 4-(4-phenoxybutoxy)furo[3,2-g]chromen-7-one |
| 4-(4-Phenoxybutoxy)-7H-furo[3,2-g]chromen-7-one |
| 5-(4-Phenoxybutoxy)psoralen |
| PAP-1 |