Leptomycin B
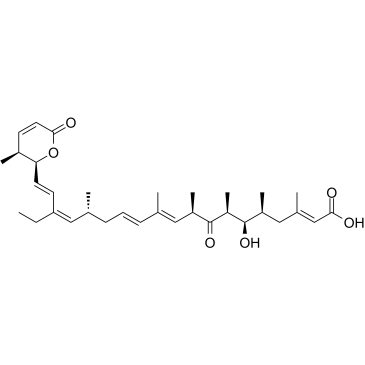
Leptomycin B structure
|
Common Name | Leptomycin B | ||
|---|---|---|---|---|
| CAS Number | 87081-35-4 | Molecular Weight | 540.73 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 725.8±60.0 °C at 760 mmHg | |
| Molecular Formula | C33H48O6 | Melting Point | 41-44ºC | |
| MSDS | N/A | Flash Point | 224.8±26.4 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Leptomycin BLeptomycin B (CI 940; LMB) is a potent inhibitor of the nuclear export of proteins. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue. Leptomycin B is a potent antifungal antibiotic blocking the eukaryotic cell cycle[1]. |
| Name | leptomycin B |
|---|---|
| Synonym | More Synonyms |
| Description | Leptomycin B (CI 940; LMB) is a potent inhibitor of the nuclear export of proteins. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue. Leptomycin B is a potent antifungal antibiotic blocking the eukaryotic cell cycle[1]. |
|---|---|
| Related Catalog | |
| Target |
CRM1/exportin 1[1] |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 725.8±60.0 °C at 760 mmHg |
| Melting Point | 41-44ºC |
| Molecular Formula | C33H48O6 |
| Molecular Weight | 540.73 |
| Flash Point | 224.8±26.4 °C |
| Exact Mass | 540.345093 |
| PSA | 100.90000 |
| LogP | 6.66 |
| Vapour Pressure | 0.0±5.3 mmHg at 25°C |
| Index of Refraction | 1.542 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301-H311-H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | T: Toxic;F: Flammable; |
| Risk Phrases | R23/25 |
| Safety Phrases | 7-16-24-33-45 |
| RIDADR | UN 1230 3 |
| WGK Germany | 3 |
| HS Code | 2916190090 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2916190090 |
|---|---|
| Summary | 2916190090 unsaturated acyclic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:6.5%。general tariff:30.0% |
|
A survey of the interactome of Kaposi's sarcoma-associated herpesvirus ORF45 revealed its binding to viral ORF33 and cellular USP7, resulting in stabilization of ORF33 that is required for production of progeny viruses.
J. Virol. 89(9) , 4918-31, (2015) The ORF45 protein of Kaposi's sarcoma-associated herpesvirus (KSHV) is a gammaherpesvirus-specific immediate-early tegument protein. Our previous studies have revealed its crucial roles in both early ... |
|
|
African swine fever virus ORF P1192R codes for a functional type II DNA topoisomerase.
Virology 474 , 82-93, (2014) Topoisomerases modulate the topological state of DNA during processes, such as replication and transcription, that cause overwinding and/or underwinding of the DNA. African swine fever virus (ASFV) is... |
|
|
Identification of novel DNA-damage tolerance genes reveals regulation of translesion DNA synthesis by nucleophosmin.
Nat. Commun. 5 , 5437, (2014) Cells cope with replication-blocking lesions via translesion DNA synthesis (TLS). TLS is carried out by low-fidelity DNA polymerases that replicate across lesions, thereby preventing genome instabilit... |
| Leptomycin B,(2E,5S,6R,7S,9R,10E,12E,15R,16Z,18E)-19-[(2S,3S)-3,6-Dihydro-3-methyl-6-oxo-2H-pyran-2-yl]-17-ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-8-oxo-2,10,12,16,18-nonadecapentaenoicacid |
| 2,10,12,16,18-nonadecapentaenoic acid |
| 2,10,12,16,18-Nonadecapentaenoic acid, 19-[(2S,3S)-3,6-dihydro-3-methyl-6-oxo-2H-pyran-2-yl]-17-ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-8-oxo-, (2E,5S,6R,7S,9R,10E,12E,15R,16E,18E)- |
| 2,10,12,16,18-Nonadecapentaenoic acid, 19-[(2S,3S)-3,6-dihydro-3-methyl-6-oxo-2H-pyran-2-yl]-17-ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-8-oxo-, (2E,5S,6R,7S,9R,10E,12E,15R,16Z,18E)- |
| 7,9,11,15-hexamethyl-8-oxo--yl)-17-ethyl-6-hydroxy-5 |
| (2E,5S,6R,7S,9R,10E,12E,15R,16E,18E)-17-Ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-19-[(2S,3S)-3-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl]-8-oxo-2,10,12,16,18-nonadecapentaenoic acid |
| antibioticci940 |
| (2E,5S,6R,7S,9R,10E,12E,15R,16Z,18E)-17-Ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-19-[(2S,3S)-3-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl]-8-oxo-2,10,12,16,18-nonadecapentaenoic acid |
| antibioticcl1957a |
| LEPTOMYCIN B FROM STREPTOMYCES SP |
| Leptomycin B |
 CAS#:217640-04-5
CAS#:217640-04-5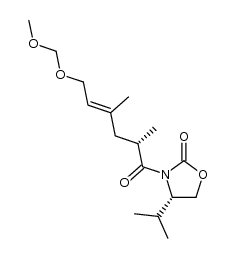 CAS#:217640-11-4
CAS#:217640-11-4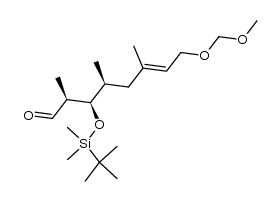 CAS#:217640-14-7
CAS#:217640-14-7
