GW 6471
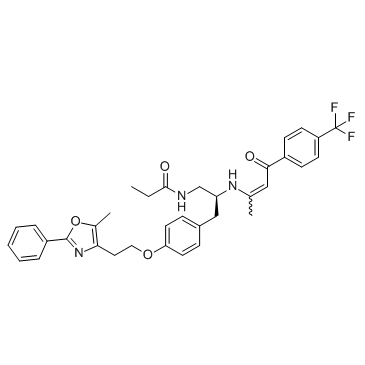
GW 6471 structure
|
Common Name | GW 6471 | ||
|---|---|---|---|---|
| CAS Number | 880635-03-0 | Molecular Weight | 619.673 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C35H36F3N3O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of GW 6471GW 6471 is a potent PPARα antagonist. |
| Name | N-[(2S)-3-[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]-2-[[(E)-4-oxo-4-[4-(trifluoromethyl)phenyl]but-2-en-2-yl]amino]propyl]propanamide |
|---|---|
| Synonym | More Synonyms |
| Description | GW 6471 is a potent PPARα antagonist. |
|---|---|
| Related Catalog | |
| Target |
PPARα |
| In Vitro | In a cell-based reporter assay, GW 6471 completely inhibits GW409544-induced activation of PPARα with an IC50 of 0.24 μM[1]. The functional role of PPARα is evaluated on renal cell carcinoma (RCC) cell viability by MTT assay. Both Caki-1 (VHL wild type) and 786-O (VHL mutated) cells are incubated separately with a specific PPARα agonist, WY14,643, or a specific PPARα antagonist, GW 6471 at concentrations from 12.5 to 100 µM for 72 hours, and cell viability is assessed. While WY14,643 either has no affect on, or slightly increased, cell viability, GW 6471 significantly and dose-dependently inhibits cell viability (up to approximately 80%) in both cell lines[2]. |
| In Vivo | To test the antitumor activity of PPARα antagonism in vivo, a subcutaneous xenograft mouse model is used. Caki-1 cells are implanted subcutaneously in nude (Nu/Nu) mice. After tumor masses reach ∼5 mm in diameter, GW 6471 is administrated intraperitoneally every other day for 4 wk at a dose (20 mg/kg mouse body wt) that is described to be effective in an in vivo dose-response study and confirmed here to be efficacious. There are significant differences in tumor growth between vehicle- and GW 6471-treated animals. No toxicity is observed at the doses of GW 6471 based on weights of the animals, and laboratory values, including kidney and liver function tests, are not adversely affected. To demonstrate on-target effects of GW 6471, c-Myc levels are evaluated in the tumors, which show significant decreases in the GW 6471-treated animals[3]. |
| Cell Assay | 786-O and Caki-1 cells are plated in 96 well plates. Both cells are incubated separately with WY14,643 or GW 6471 at concentrations from 12.5 to 100 µM for 72 hours, and after the indicated treatments, the cells are incubated in MTT solution/media mixture. Then, the MTT solution is removed and the blue crystalline precipitate in each well is dissolved in DMSO. Visible absorbance of each well at 540 nm is quantified using a microplate reader[2]. |
| Animal Admin | Mice[3] Male athymic Nu/Nu mice (8 wk of age, ~25 g body wt) are injected with 1×105 Caki-1 cells subcutaneously (3:1 DMEM-Matrigel) in the flank region. Tumor progression is monitored weekly by calipers. When tumor size reaches ~80-100 mm3, animals are randomly assigned to four groups and treatments are started (day 1). The vehicle group receive DMSO (4% in PBS) intraperitoneally and vegetable oil via oral gavage. The PPARα group is injected intraperitoneally with GW 6471 in the same vehicle (20 mg/kg body wt; murine dose response is reported elsewhere) every other day. The Sunitinib group receive Sunitinib in vegetable oil via oral gavage (40 mg/kg body wt) 5 days/wk. Another group receive GW 6471+Sunitinib. To determine any potential toxicity of the treatment(s), body weights of the animals are measured and signs of adverse reactions are monitored. On day 28, the mice are euthanized and the tumor mass is determined. Tumor growth rate is calculated[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C35H36F3N3O4 |
| Molecular Weight | 619.673 |
| Exact Mass | 619.265808 |
| PSA | 96.95000 |
| LogP | 7.24 |
| Index of Refraction | 1.556 |
| InChIKey | TYEFSRMOUXWTDN-DYQICHDWSA-N |
| SMILES | CCC(=O)NCC(Cc1ccc(OCCc2nc(-c3ccccc3)oc2C)cc1)NC(C)=CC(=O)c1ccc(C(F)(F)F)cc1 |
| Storage condition | 2-8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| RIDADR | NONH for all modes of transport |
|
Palmitoylethanolamide controls reactive gliosis and exerts neuroprotective functions in a rat model of Alzheimer's disease.
Cell Death Dis. 5 , e1419, (2014) Given the complex heterogeneity of pathological changes occurring in Alzheimer's disease (AD), any therapeutic effort absolutely requires a multi-targeted approach, because attempts addressing only a ... |
|
|
Influence of dietary sugar on cholesterol and bile acid metabolism in the rat: Marked reduction of hepatic Abcg5/8 expression following sucrose ingestion.
Biochem. Biophys. Res. Commun. 461 , 592-7, (2015) Previous studies have indicated that dietary intake of sugar may lower bile acid production, and may promote cholesterol gallstone formation in humans. We studied the influence of dietary sucrose on c... |
|
|
Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1.
Br. J. Pharmacol. 173 , 545-61, (2016) Sepsis is a systemic inflammatory response accompanied by excessive production of inflammatory cytokines and cardiovascular dysfunction. Importantly, macrophage-derived pro-inflammatory agents play a ... |
| Propanamide, N-[(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propen-1-yl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]propyl]- |
| N-[(2S)-3-{4-[2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl}-2-({(2Z)-4-oxo-4-[4-(trifluoromethyl)phenyl]-2-buten-2-yl}amino)propyl]propanamide |