Ebastine
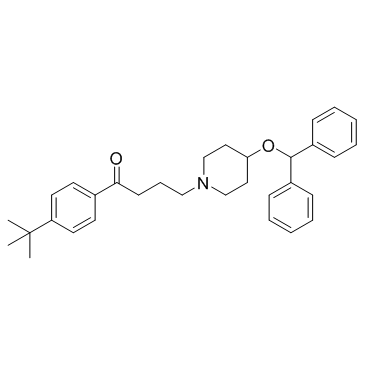
Ebastine structure
|
Common Name | Ebastine | ||
|---|---|---|---|---|
| CAS Number | 90729-43-4 | Molecular Weight | 469.658 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 596.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C32H39NO2 | Melting Point | 80-82°C | |
| MSDS | Chinese USA | Flash Point | 314.5±30.1 °C | |
Use of EbastineEbastine(LAS-W 090;RP64305) is a long-acting and selective H1-histamine receptor antagonist.Target: Histamine H1 ReceptorEbastine is a H1 antihistamine with low potential for causing drowsiness. Ebastine (10 mg orally) causes brain histamine H1-receptor occupation of approximately 10%, consistent with its lower incidence of sedative effect, whereas (+)-chlorpheniramine occupied about 50% of brain H1-receptors even at a low but sedative dose of 2 mg; occupancy of (+)-chlorpheniramine was correlated with plasma (+)-chlorpheniramine concentration [1]. ebastine 10 or 20 mg once daily was rapidly effective in relieving symptoms of PAR in adult and adolescent patients; additional benefits of the 20-mg dose became apparent in the longer term [2]. ebastine is an effective and generally well-tolerated treatment for allergic rhinitis and chronic idiopathic urticaria. In addition to the regular tablet formulation, ebastine is available as a FDT, providing a treatment option that is particularly convenient for patients [3]. |
| Name | 4-(4-benzhydryloxypiperidin-1-yl)-1-(4-tert-butylphenyl)butan-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | Ebastine(LAS-W 090;RP64305) is a long-acting and selective H1-histamine receptor antagonist.Target: Histamine H1 ReceptorEbastine is a H1 antihistamine with low potential for causing drowsiness. Ebastine (10 mg orally) causes brain histamine H1-receptor occupation of approximately 10%, consistent with its lower incidence of sedative effect, whereas (+)-chlorpheniramine occupied about 50% of brain H1-receptors even at a low but sedative dose of 2 mg; occupancy of (+)-chlorpheniramine was correlated with plasma (+)-chlorpheniramine concentration [1]. ebastine 10 or 20 mg once daily was rapidly effective in relieving symptoms of PAR in adult and adolescent patients; additional benefits of the 20-mg dose became apparent in the longer term [2]. ebastine is an effective and generally well-tolerated treatment for allergic rhinitis and chronic idiopathic urticaria. In addition to the regular tablet formulation, ebastine is available as a FDT, providing a treatment option that is particularly convenient for patients [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 596.3±50.0 °C at 760 mmHg |
| Melting Point | 80-82°C |
| Molecular Formula | C32H39NO2 |
| Molecular Weight | 469.658 |
| Flash Point | 314.5±30.1 °C |
| Exact Mass | 469.298065 |
| PSA | 29.54000 |
| LogP | 7.79 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.590 |
| InChIKey | MJJALKDDGIKVBE-UHFFFAOYSA-N |
| SMILES | CC(C)(C)c1ccc(C(=O)CCCN2CCC(OC(c3ccccc3)c3ccccc3)CC2)cc1 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Statements | H413 |
|---|---|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| RIDADR | NONH for all modes of transport |
| RTECS | EL8140000 |
|
~56% 
Ebastine CAS#:90729-43-4 |
| Literature: Arevipharma GmbH Patent: EP2371817 A1, 2011 ; Location in patent: Page/Page column 27 ; |
|
~% 
Ebastine CAS#:90729-43-4 |
| Literature: WO2011/121099 A2, ; Page/Page column 42 ; |
|
~66% 
Ebastine CAS#:90729-43-4 |
| Literature: AREVIPHARMA GMBH; SCHICKANEDER, Christian; MCGRATH, Matthew; SCHAeFER, Juergen; SCHUBERT, Jana; JACOB, Ulrike; MAeRTENS, Welljanne Patent: WO2011/121099 A2, 2011 ; Location in patent: Page/Page column 40-41 ; |
|
~% 
Ebastine CAS#:90729-43-4 |
| Literature: WO2011/121099 A2, ; Page/Page column 49 ; |
|
~% 
Ebastine CAS#:90729-43-4 |
| Literature: WO2012/76919 A1, ; |
|
~% 
Ebastine CAS#:90729-43-4 |
| Literature: WO2012/76919 A1, ; |
|
Anaphylaxis to oral iron salts. desensitization protocol for tolerance induction.
J. Investig. Allergol. Clin. Immunol. 18(4) , 305-8, (2008) Allergies to iron salts are seldom reported. We studied a patient with iron-deficiency anemia who had suffered anaphylactic reactions caused by oral iron salts. An allergy study was performed using si... |
|
|
A novel approach for predicting P-glycoprotein (ABCB1) inhibition using molecular interaction fields.
J. Med. Chem. 54 , 1740-51, (2011) P-glycoprotein (Pgp or ABCB1) is an ABC transporter protein involved in intestinal absorption, drug metabolism, and brain penetration, and its inhibition can seriously alter a drug's bioavailability a... |
|
|
New therapies for allergic rhinitis.
Curr. Allergy Asthma Rep. 14(4) , 422, (2014) Because of its burden on patient's lives and its impact on asthma, allergic rhinitis must be treated properly with more effective and safer treatments. According to guidelines by Allergic Rhinitis and... |
| 4-Diphenylmethoxy-1-[3-(4-tert-butylbenzoyl)propyl]piperidine |
| 4'-tert-Butyl-4-[4-(diphenylmethoxy)piperidino]butyrophenone |
| Ebastinum [Latin] |
| [14C]-Ebastine |
| carebastine |
| 4-diphenylmethoxy-1-[3-(4-tert-butylbenzoyl)propyl]-piperidine |
| Ebastina [Spanish] |
| 4-[4-(Diphenylmethoxy)-1-piperidinyl]-1-[4-(2-methyl-2-propanyl)phenyl]-1-butanone |
| Kestine |
| LAS W-090 |
| 1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piperidin-1-yl]butan-1-one |
| MFCD00865661 |
| Ebastel |
| 1-Butanone, 1-[4-(1,1-dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]- |
| 1-[4-(1,1-Dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]-1-butanone |
| Ebastin |
| Ebastine |
| Estivan |
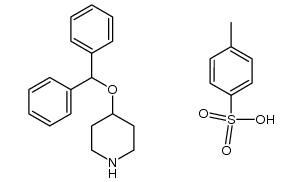


![1-[4-(1,1-dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]-1-butanone tosylate structure](https://image.chemsrc.com/caspic/423/1338066-82-2.png)
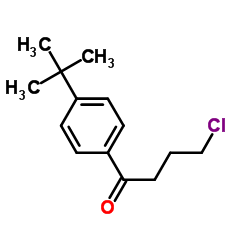
 CAS#:210686-41-2
CAS#:210686-41-2
