Lorazepam glucuronide
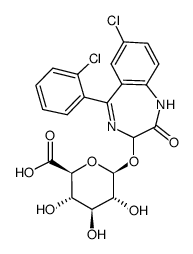
Lorazepam glucuronide结构式

|
常用名 | Lorazepam glucuronide | 英文名 | Lorazepam glucuronide |
|---|---|---|---|---|
| CAS号 | 32781-79-6 | 分子量 | 497.28200 | |
| 密度 | 1.76g/cm3 | 沸点 | 779.5ºC at 760mmHg | |
| 分子式 | C21H18Cl2N2O8 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | 425.2ºC | |
| 符号 |


GHS02, GHS07 |
信号词 | Danger |
|
Commentary on: Dou C, Bournique J, Zinda M, Gnezda M, Nally A, Salamone S. Comparison of rates of hydrolysis of lorazepam-glucuronide, oxazepam-glucuronide and temazepam-glucuronide catalyzed by E. coli beta-glucuronidase using the on-line benzodiazepine screening immunoassay on the Roche/Hitachi 917 analyzer.
J. Forensic Sci. 47(2) , 427-8, (2002)
|
|
|
Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration.
Am. J. Kidney Dis. 45(2) , 360-71, (2005) The objective is to study the population pharmacokinetics of lorazepam and midazolam in critically ill patients with acute renal failure who are treated with continuous venovenous hemofiltration (CVVH).Twenty critically ill patients with acute renal failure o... |
|
|
Biotransformation and excretion of lorazepam in patients with chronic renal failure.
Br. J. Clin. Pharmacol. 3(6) , 1033-9, (1976) To evaluate the effect of end-stage renal insufficiency and haemodialysis on the elimination of lorazepam, single oral doses of the drug (2.5 mg) were administered to normal subjects and patients with chronic renal failure (CC(r) : less than 2 ml/min) in the ... |
|
|
Analysis of lorazepam and its 30-glucuronide in human urine by capillary electrophoresis: evidence for the formation of two distinct diastereoisomeric glucuronides.
J. Sep. Sci. 29(1) , 153-63, (2006) Lorazepam (LOR) is a 3-hydroxy-1,4-benzodiazepine that is chiral and undergoes enantiomerization at room temperature. In humans, about 75% of the administered dose of LOR is excreted in the urine as its 30-glucuronide. CE-MS with negative ESI was used to conf... |
|
|
Quantitative assay of lorazepam and its metabolite glucuronide by reverse-phase liquid chromatography-tandem mass spectrometry in human plasma and urine samples.
J. Pharm. Biomed. Anal. 40(2) , 389-96, (2006) A LC/MS/MS method for the quantitative determination of lorazepam in human plasma and urine samples was developed and validated. The enantioselective assay allowed to separate the enantiomers and to verify the stereochemical instability of lorazepam. The line... |
|
|
Effect of renal impairment and hemodialysis on lorazepam kinetics.
Clin. Pharmacol. Ther. 35(5) , 646-52, (1984) The effect of renal disease on lorazepam kinetics was assessed in three groups: six normal subjects, six patients with renal impairment, and four functionally anephric patients. Subjects received single 1.5- or 3-mg intravenous and oral doses; eight subjects ... |
|
|
Kinetic disposition of lorazepam with focus on the glucuronidation capacity, transplacental transfer in parturients and racemization in biological samples.
J. Pharm. Biomed. Anal. 40(2) , 397-403, (2006) The present study investigates the kinetic disposition with focus on the racemization, glucuronidation capacity and the transplacental transfer of lorazepam in term parturients during labor. The study was conducted on 10 healthy parturients aged 18-37 years w... |
|
|
Periportal localization of lorazepam glucuronidation in the isolated perfused rat liver.
J. Lab. Clin. Med. 102(5) , 805-12, (1983) The relative effects of pretreatment with allyl alcohol and carbon tetrachloride on oxidative and glucuronide metabolism of lorazepam have been compared in the isolated perfused rat liver. Livers from rats pretreated for 24 hr with allyl alcohol (1.8 ml/kg, 1... |
|
|
A study of conjugation and drug elimination in the human neonate.
Br. J. Clin. Pharmacol. 12(4) , 511-5, (1981) 1 An investigation has been made of the excretion of alpha-methyldopa, alpha-methyldopa sulphate, lorazepam and lorazepam glucuronide in the urine of neonates. 2 The rate of elimination of both the drugs in the newborn is slow compared with the adult rate, an... |
|
|
In vitro binding of lorazepam and lorazepam glucuronide to cholestyramine, colestipol, and activated charcoal.
Pharm. Res. 8(4) , 538-40, (1991)
|