
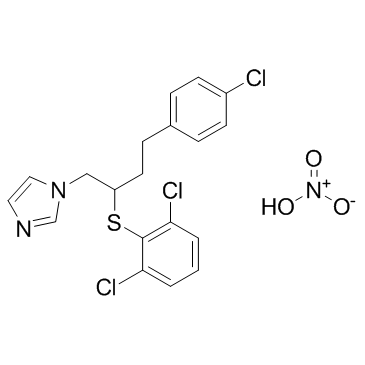
硝酸布康唑结构式

|
常用名 | 硝酸布康唑 | 英文名 | Butoconazole nitrate |
|---|---|---|---|---|
| CAS号 | 64872-77-1 | 分子量 | 474.789 | |
| 密度 | N/A | 沸点 | 566.9ºC at 760 mmHg | |
| 分子式 | C19H18Cl3N3O3S | 熔点 | 159ºC (dec.) | |
| MSDS | 中文版 美版 | 闪点 | 296.7ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Effects of terconazole and other azole antifungal agents on the sterol and carbohydrate composition of Candida albicans.
Diagn. Microbiol. Infect. Dis. 13(1) , 31-5, (1990) The effects of terconazole, a triazole antifungal, on the sterol and carbohydrate composition of Candida albicans was compared with that of three imidazoles: clotrimazole, miconazole, and butoconazole. Exposure of C. albicans to terconazole resulted in a prof... |
|
|
Effect of butoconazole nitrate cream and wax insert on the barrier property of contraceptive devices.
Am. J. Obstet. Gynecol. 158(4) , 1011-3, (1988) A new method was developed to assess the quality of the barrier property of several types of commonly used contraceptive devices (diaphragm, cervical cap, and condom). Our data showed that butoconazole nitrate cream and wax inserts did not affect the barrier ... |
|
|
Patient acceptance of prefilled disposable vaginal applicator.
Am. J. Obstet. Gynecol. 158(4) , 1006-8, (1988) Patient acceptance of a new, compact, prefilled disposable vaginal applicator was tested in an open study in which 2% butoconazole cream was used for the treatment of vulvovaginal candidiasis. Twenty nonpregnant patients with clinical signs and symptoms of ca... |
|
|
Control of Candida albicans vaginitis in mice by short-duration butoconazole treatment in situ.
Mycoses 36(11-12) , 379-84, (1993) A short-duration treatment for candidal vaginitis applying butoconazole in situ was tested in an experimental mouse model. One week after artificial induction of an oestrus state (by oestradiol benzoate injection), mice were inoculated intravaginally with 1.5... |
|
|
Vaginal retention of 2% butoconazole nitrate cream: comparison of a standard and a sustained-release preparation.
Clin. Ther. 16(6) , 930-4, (1994) The purpose of this study was to establish the length of time that butoconazole nitrate 2% standard cream and sustained-release cream could be detected in the vagina after a single administration of 5 g of cream that contained 100 mg of butoconazole. Sixteen ... |
|
|
Vulvovaginal candidosis: comparison of 3-day treatment with 2% butoconazole nitrate cream and 6-day treatment with 1% clotrimazole cream.
J. Int. Med. Res. 16(5) , 367-75, (1988) Sixty-three women with laboratory confirmed diagnoses of vulvovaginal candidosis were enrolled into this randomized, single-blind, parallel comparison of treatment with 2% butoconazole nitrate cream for 3 days and 1% clotrimazole cream for 6 days. Approximate... |
|
|
Drugs for vulvovaginal candidiasis.
Med. Lett. Drugs Ther. 43(1095) , 3-4, (2001)
|
|
|
[In vitro evaluation of the activity of butoconazole against Trichomonas vaginalis].
Pathol. Biol. 40(5) , 492-4, (1992) An in vitro study was carried out on ten first clinical isolates of Trichomonas vaginalis. Increasing levels of butaconazole were added to 150 microliters of a dilution in Roiron medium adjusted to 50,000 Trichomonas/ml. Results were read after 1, 2, 6 and 24... |
|
|
A comparison of butoconazole nitrate cream with econazole nitrate cream for the treatment of vulvovaginal candidiasis.
J. Int. Med. Res. 18(5) , 389-99, (1990) In a randomized, single-blind, parallel study the safety and efficacy of 2% butoconazole nitrate cream used for 3 days were compared with those of 1% econazole nitrate cream used for 7 days at night in patients with vulvovaginal candidiasis. Patients in both ... |
|
|
Butoconazole nitrate 2% for vulvovaginal candidiasis. New, single-dose vaginal cream formulation vs. seven-day treatment with miconazole nitrate. Gynazole 1 Study Group.
J. Reprod. Med. 44(11) , 933-8, (1999) To compare the safety and efficacy of a single vaginal dose of a butoconazole nitrate 2% bioadhesive, sustained-release cream* (butoconazole 1-BSR) with a seven-day schedule of miconazole nitrate vaginal cream 2% (miconazole 7).The clinical trial was conducte... |