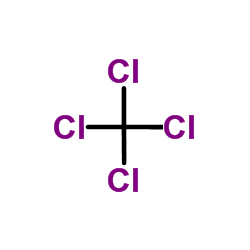
56-23-5
| 中文名 | 四氯化碳 |
|---|---|
| 英文名 | tetrachloromethane |
| 中文别名 |
四氯甲
四氯甲烷 |
| 英文别名 |
tert-butyl N-acetate
EINECS 203-453-4 tetrachloro-methane 1,1-Dimethyl acetate tert-Butyl ethanoate anhydrous tert-butyl acetate Carbon tetrachloride tert-BuOCOCH3 carbone tetrachloride acetic acid tert-butyl ester anhydrous tetra-chloro-methane carbon chloride Acetic acid,1,1-dimethylethyl ester t-Butyl acetate Texaco lead appreciator 1,1-dimethylethyl acetate MFCD00006998 |
| 描述 | Carbon tetrachloride 是一种无色不燃液体。Carbon tetrachloride 通过交感神经通路诱导急性肝损伤。Carbon tetrachloride 引起肝纤维化和肝毒性。Carbon tetrachloride 具有口服活性。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.7±0.1 g/cm3 |
|---|---|
| 沸点 | 76.0±8.0 °C at 760 mmHg |
| 熔点 | -23 °C |
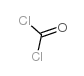
| 分子式 | CCl4 |
| 分子量 | 153.82 |
| 闪点 | -0.3±15.8 °C |
| 精确质量 | 151.875412 |
| LogP | 2.86 |
| 外观性状 | 无色有特臭的透明液体,极易挥发。 |
| 蒸汽密度 | 5.32 (vs air) |
| 蒸汽压 | 112.7±0.1 mmHg at 25°C |
| 折射率 | 1.487 |
| 储存条件 | 储存注意事项 储存于阴凉、通风的库房。远离火种、热源。库温不超过32℃,相对湿度不超过80%。保持容器密封。应与氧化剂、活性金属粉末、食用化学品分开存放,切忌混储。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 稳定性 | 1.稳定性 稳定 2.禁配物 活性金属粉末、强氧化剂 3.避免接触的条件 潮湿空气、光照 4.聚合危害 不聚合 |
| 水溶解性 | 0.8 g/L (20 ºC) |
| 分子结构 | 1、摩尔折射率:26.04 2、摩尔体积(cm3/mol):90.6 3、等张比容(90.2K):220.7 4、表面张力(dyne/cm):35.2 5、极化率(10-24cm3):10.32 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):2.8 2.氢键供体数量:0 3.氢键受体数量:0 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积0 7.重原子数量:5 8.表面电荷:0 9.复杂度:19.1 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:无色透明易挥发液体,有特殊的芳香气味。 2. 沸点(ºC,101.3kPa):76.72 3. 熔点(ºC):-22.92 4. 相对密度(g/mL,20/4ºC):1.5942 5. 折射率(n20ºC):1.4604 6. 黏度(mPa·s,20ºC):0.965 7. 蒸发热(KJ/mol,b.p.):29.982 8. 熔化热(KJ/mol):2.433 9. 生成热(KJ/mol,25ºC,液体):135.5 10. 燃烧热(KJ/mol,25ºC,液体):258.24 11. 比热容(KJ/(kg·K),20ºC):0.866 12. 临界温度(ºC):283.15 13. 临界压力(MPa):4.56 14. 沸点上升常数:4.88 15. 电导率(S/m,18ºC):4×10-18 16. 体膨胀系数(K-1,20ºC):0.00127 17. 溶解性:能与水、醇、醚、石油醚、石脑油、冰乙酸、二硫化碳、氯代烃等大多数有机溶剂混溶。 18. 相对密度(25℃,4℃):1.5846 19. 常温折射率(n25):1.4573 20. 蒸气压(kPa,20ºC):11.9102 21. 临界密度(g·cm-3):0.557 22. 临界体积(cm3·mol-1):276 23. 临界压缩因子:0.272 24. 偏心因子:0.193 25.Lennard-Jones参数(A):7.949 26.Lennard-Jones参数(K):633.6 27.溶度参数(J·cm-3)0.5:17.577 28.van der Waals面积(cm2·mol-1):7.280×109 29.van der Waals体积(cm3·mol-1):52.300 30.气相标准声称热(焓)( kJ·mol-1) :-95.8 31.气相标准熵(J·mol-1·K-1) :310.02 32.气相标准生成自由能( kJ·mol-1):-53.5 33.气相标准热熔(J·mol-1·K-1):83.43 34.液相标准声称热(焓)( kJ·mol-1):-128.41 35.液相标准熵(J·mol-1·K-1) :216.19 36.液相标准生成自由能( kJ·mol-1):-60.50 37.液相标准热熔(J·mol-1·K-1):131.4 |
Synonym: Tetrachloromethane; Carbon tet; Carbona; Carbon chloride; Methane tetrachloride. SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 23/24/25 40 59 48/23 52/53 SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Toxic by inhalation, in contact with skin and if swallowed. Limited evidence of a carcinogenic effect. Dangerous for the ozone layer. Toxic : danger of serious damage to health by prolonged exposure through inhalation. Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment.Cancer suspect agent. Potential Health Effects Eye: Causes eye irritation. Vapors cause eye irritation. Skin:
Causes skin irritation. May be absorbed through the skin in harmful amounts. Contact with the skin defats the skin. Ingestion: May cause liver and kidney damage. May cause central nervous system depression, characterized by excitement, followed by headache, dizziness, drowsiness, and nausea. Advanced stages may cause collapse, unconsciousness, coma and possible death due to respiratory failure. Substance is a hepatotoxin and is capable of producing a toxic effect on the liver. Inhalation: May cause liver and kidney damage. Exposure produces central nervous system depression. May be harmful if inhaled. Chronic: Prolonged or repeated skin contact may cause dermatitis. Chronic ingestion may cause effects similar to those of acute ingestion. May cause liver and kidney damage. May cause cancer according to animal studies. Chronic exposure may cause visual disturbances. Carbon tetrachloride is a CNS depressant. SECTION 4 - FIRST AID MEASURES Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid immediately. Wash clothing before reuse. Ingestion: Potential for aspiration if swallowed. Get medical aid immediately. Do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Inhalation: Poison material. If inhaled, get medical aid immediately. Remove victim to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Notes to Physician: SECTION 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Material will not burn. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Extinguishing Media: Use extinguishing media most appropriate for the surrounding fire. SECTION 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Isolate area and deny entry. Provide ventilation. SECTION 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Do not breathe vapor. Use only with adequate ventilation. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use only under a chemical fume hood. Personal Protective Equipment Eyes: Wear chemical goggles. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary. SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Color: clear, colorless Odor: chloroform-like pH: Not available. Vapor Pressure: 91 mm Hg @ 20 deg C Viscosity: 0.97 PAS 20 deg C Boiling Point: 76 deg C @ 760 mm Hg Freezing/Melting Point: -23 deg C Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: >100 deg C Solubility in water: Insoluble. Specific Gravity/Density: 1.5900 g/cm3 Molecular Formula: CCl4 Molecular Weight: 153.82 SECTION 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Light, excess heat. Incompatibilities with Other Materials: Alkali metals, powdered aluminum, powdered magnesium, zinc powder, ethylene, allyl alcohol, barium, fluorine, dimethylformamide, powered beryllium, decaborane, potassium tert-butoxide. Hazardous Decomposition Products: Hydrogen chloride, chlorine, phosgene, carbon monoxide, carbon dioxide, chlorine dioxide, which may be spontaneously explosive. Hazardous Polymerization: Will not occur. SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 56-23-5: FG4900000 LD50/LC50: CAS# 56-23-5: Dermal, guinea pig: LD50 = >9400 uL/kg; Draize test, rabbit, eye: 2200 ug/30S Mild; Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 4 mg Mild; Draize test, rabbit, skin: 500 mg/24H Mild; Inhalation, mouse: LC50 = 9526 ppm/8H; Inhalation, rat: LC50 = 8000 ppm/4H; Oral, mouse: LD50 = 8263 mg/kg; Oral, rabbit: LD50 = 5760 mg/kg; Oral, rat: LD50 = 2350 mg/kg; Skin, rabbit: LD50 = >20 gm/kg; Skin, rat: LD50 = 5070 mg/kg. Carcinogenicity: Carbon Tetrachloride - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA. Other: See actual entry in RTECS for complete information. SECTION 12 - ECOLOGICAL INFORMATION Ecotoxicity: Fish: Fathead Minnow: LC50 = 20.8-41.4 mg/L; 96 Hr.; Flow-through; 21.7 degrees CFish: Bluegill/Sunfish: LC50 = 27-125 mg/L; 96 Hr.; Static Conditions; 23 degrees CBacteria: Phytobacterium phosphoreum: EC50 = 6.0 mg/L; Not available; Microtox testBacteria: Phytobacterium phosphoreum: EC50 = 33.0 mg/L; 30 minutes; Microtox test SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. SECTION 14 - TRANSPORT INFORMATION IATA Shipping Name: CARBON TETRACHLORIDE Hazard Class: 6.1 UN Number: 1846 Packing Group: II IMO Shipping Name: CARBON TETRACHLORIDE Hazard Class: 6.1 UN Number: 1846 Packing Group: II RID/ADR Shipping Name: CARBON TETRACHLORIDE Hazard Class: 6.1 UN Number: 1846 Packing group: II SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: T N Risk Phrases: R 23/24/25 Toxic by inhalation, in contact with skin and if swallowed. R 40 Limited evidence of a carcinogenic effect. R 59 Dangerous for the ozone layer. R 48/23 Toxic : danger of serious damage to health by prolonged exposure through inhalation. R 52/53 Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment. Safety Phrases: S 23 Do not inhale gas/fumes/vapour/spray. S 36/37 Wear suitable protective clothing and gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). S 59 Refer to manufacturer/supplier for information on recovery/recycling. S 61 Avoid release to the environment. Refer to special instructions/Safety data sheets. WGK (Water Danger/Protection) CAS# 56-23-5: 3 United Kingdom Occupational Exposure Limits United Kingdom Maximum Exposure Limits Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 56-23-5 is not listed on Canada's Ingredient Disclosure List. Exposure Limits CAS# 56-23-5: OEL-ARAB Republic of Egypt:TWA 5 ppm (30 mg/m3);Skin OEL-AUSTRALIA:TWA 5 ppm (30 mg/m3);Skin;Carcinoge OEL-BELGIUM:TWA 5 ppm (31 mg/m3);Skin;Carcinogen OEL-CZECHOSLOVAKIA:TWA 10 mg/m3;STEL 20 mg/m3 OEL-DENMARK:TWA 2 ppm (13 mg/m3);Skin OEL-FINLAND:TWA 5 ppm (31 mg/m3);STEL 10 ppm (63 mg/m3);Skin;CAR OEL-FRANCE:TWA 2 ppm (12 mg/m3);STEL 10 ppm (60 mg/m3) OEL-GERMANY:TWA 10 ppm (65 mg/m3);Skin;Carcinogen OEL-HUNGARY:STEL 10 mg/m3;Skin;Carcinogen OEL-INDIA:TWA 5 ppm (30 mg/m3);Skin;Carcinogen OEL-JAPAN:TWA 10 ppm (63 mg/m3);Skin;Carcinogen OEL-THE NETHERLANDS:TWA 2 ppm (12.6 mg/m3);Skin OEL-THE PHILIPPINES:TWA 10 ppm (65 mg/m3);Skin OEL-POLAND:TWA 20 mg/m3 OEL-RUSSIA:TWA 10 ppm;STEL 20 mg/m3 OEL-SWEDEN:TWA 2 ppm (13 mg/m3);STEL 3 ppm (19 mg/m3);Skin;CAR OEL-SWITZERLAND:TWA 5 ppm (30 mg/m3);STEL 10 ppm (60 mg/m3);Skin OEL-THAILAND:TWA 10 ppm;STEL 25 ppm OEL-UNITED KINGDOM:TWA 10 ppm (65 mg/m3);STEL 20 ppm;Skin OEL IN BULGARIA, COLOMBIA, JORDAN, KOREA check ACGIH TLV OEL IN NEW ZEALAND, SINGAPORE, VIETNAM check ACGI TLV US FEDERAL TSCA CAS# 56-23-5 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION MSDS Creation Date: 7/20/1999 Revision #4 Date: 11/06/2002 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 1.急性毒性 LD50:2350mg/kg(大鼠经口);5070mg/kg(大鼠经皮) LC50:50400mg/m3(大鼠吸入,4h) 2.刺激性 家兔经皮:4mg,轻度刺激。 家兔经眼:500mg(24h),轻度刺激。 3.亚急性与慢性毒性 长期低剂量接触本品的主要损害肝和肾。 4.致突变性 微生物致突变:鼠伤寒沙门菌20μl/L。DNA损伤:小鼠经口335μmol/kg。性染色体缺失和不分离:仓鼠肺1600μmol/L。程序外DNA合成:小鼠经口100mg/kg。DNA抑制:小鼠经口2g/kg。 5.致畸性 大鼠孕后6~15d吸入最低中毒剂量(TCLo)300ppm(7h)致肌肉骨骼系统发育畸形。大鼠孕后18d胃肠外给予最低中毒剂量(TCLo)2384mg/kg,致肝胆管系统发育畸形。 6.致癌性 IARC致癌性评论:G2B,可疑人类致癌物。 7.其他 大鼠经口最低中毒剂量(TDLo):2g/kg(孕7~8d),引起植入后死亡率增加。大鼠经口最低中毒剂量(TDLo)3691mg/kg(雄性,10d),引起睾丸、附睾和输精管异常。 生态学数据: 1.生态毒性 LC50:27~125mg/L(96h)(蓝鳃太阳鱼);20.8~41.4mg/L(96h)(黑头呆鱼);45mg/L(96h)(绿藻) IC50:600mg/L(72h)(藻类) 2.生物降解性 好氧生物降解(h):4032~8640 厌氧生物降解(h):168~672 3.非生物降解性 空气中光氧化半衰期(h):1.60×104~1.60×105 一级水解半衰期(h):7000 4.其他有害作用 四氯化碳属高蓄积性物,在哺乳动物的肝部可产生蓄积,对鲑鱼可致肝癌。该物质臭氧消耗潜能(ODP)为1.1,对大气臭氧层破坏力极强。
|
| 符号 |


GHS06, GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H301 + H311 + H331-H317-H351-H372-H412-H420 |
| 警示性声明 | P261-P273-P280-P301 + P310 + P330-P403 + P233-P502 |
| 个人防护装备 | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| 危害码 (欧洲) | T:Toxic;N:Dangerousfortheenvironment; |
| 风险声明 (欧洲) | R23/24/25;R40;R48/23;R52/53;R59 |
| 安全声明 (欧洲) | S23-S36/37-S45-S59-S61 |
| 危险品运输编码 | UN 1846 6.1/PG 2 |
| WGK德国 | 3 |
| RTECS号 | FG4900000 |
| 包装等级 | II |
| 危险类别 | 6.1(a) |
| 海关编码 | 2903140090 |
| 上游产品 10 | |
|---|---|
| 下游产品 10 | |
| 海关编码 | 2903140090 |
|---|