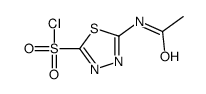
59-66-5
| 中文名 | 乙酰唑胺 |
|---|---|
| 英文名 | acetazolamide |
| 中文别名 |
2-乙酰氨基-1,3,4-噻二唑-5-磺酰胺
ACET偶氮酰胺 N-[5-(氨磺酰基)-1,3,4-噻二唑-2-基]乙酰胺 阿昔洛韦杂质A 醋唑磺胺 |
| 英文别名 |
Cidamex
Diamox N-[5-(Aminosulfonyl)-1,3,4-thiadiozol-2-yl]-Acetamide DIACARB Glupax Donmox Diluran N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide 5-acetamido-1,3,4-thiadiazole-2-sulphonamide EINECS 200-440-5 acetazolamide Atenezol MFCD00003105 Didoc Edemox Diakarb |
| 描述 | Acetazolamide 是一种碳酸酐酶 (carbonic anhydrase,CA) IX 抑制剂,抑制 hCA IX,IC50 为 30 nM[1]。利尿剂[4]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 乙酰唑胺还抑制hCA II,IC50为130 nM [1]。乙酰唑胺(Ace)是一种小的杂芳族磺酰胺,以高亲和力结合各种碳酸酐酶,充当碳酸酐酶(CA)抑制剂[2]。与对照组相比,高乙酰唑胺浓度(AceH,50 nM),顺铂(顺式;1μg/ mL)和顺式联合低乙酰唑胺浓度(AceL,10 nM)处理显着降低Hep-2细胞的活力[ 2]。用乙酰唑胺/ Cis组合治疗显着增加P53的表达水平,因为与对照组相比,AceL + Cis和AceH + Cis处理均导致P53蛋白表达水平显着增加。与对照组相比,Ace/Cis组合处理显着降低bcl-2/bax表达比率,并增加胱天蛋白酶-3蛋白的表达。与对照组相比,AceL,AceH,Cis和AceL + Cis处理显着降低bcl-2/bax比例[2]。联合Ace和Cis治疗有效促进Hep-2细胞的凋亡[2]。与Ace/Cis联合治疗显着降低Hep-2细胞中AQP1 mRNA的表达。与对照组相比,AceH和AceL + Cis处理均降低Hep-2细胞中水通道蛋白-1(AQP1)mRNA的表达[2]。 |
| 体内研究 | 乙酰唑胺(40mg/kg)显着增强MS-275对神经母细胞瘤(NB)SH-SY5Y异种移植物中肿瘤发生的抑制作用[3]。乙酰唑胺(40mg/kg)和/或MS-275处理降低NB SH-SY5Y异种移植物中HIF1-α和CAIX的表达[3]。乙酰唑胺(40mg/kg),MS-275和乙酰唑胺+ MS-275降低NB SH-SY5Y异种移植物中有丝分裂和增殖标志物的表达[3]。 |
| 细胞实验 | 细胞活力测定[2]细胞系:Hep-2细胞和HUVEC浓度:10nM和50nM孵育时间:48小时测定:通过MTT测定法测量Hep-2细胞和HUVEC的细胞活力。将处于对数生长期的Hep-2细胞和HUVEC接种在96孔板中。如所示的药物处理48小时后,向每个孔中加入200μLMTT(5mg / mL)。将细胞与MTT溶液在37℃下孵育4小时。然后,加入150μLDMSO5分钟。用Versamax Microplate读数器在490nm处测量光密度(OD)值。注意:联合治疗有效降低了Hep-2细胞的活力。 |
| 动物实验 | 体内研究[3]动物模型:4-6周龄雌性NOD / SCID小鼠剂量:40mg / kg,腹膜内注射,每天2周给药:将小鼠随机分成4组(每组5只小鼠)。对照组和治疗组分别每天腹膜内注射载体(PBS)或乙酰唑胺(40mg / kg),MS-275(20mg / kg)或组合,持续2周。当肿瘤体积超过2立方厘米或动物显示出发病迹象时终止实验。每天测量肿瘤直径直至终止。注意:抑制NB异种移植物的肿瘤生长,具有显着的抗肿瘤生长增强作用。 |
| 参考文献 |
| 密度 | 1.7±0.1 g/cm3 |
|---|---|
| 熔点 | 256-261°C |
| 分子式 | C4H6N4O3S2 |
| 分子量 | 222.245 |
| 精确质量 | 221.988129 |
| PSA | 151.66000 |
| LogP | -0.26 |
| 外观性状 | 白色至灰白色结晶粉末 |
| 折射率 | 1.641 |
| 储存条件 | 密封保存。 |
| 水溶解性 | <0.1 g/100 mL at 22 ºC |
| 分子结构 | 五、分子性质数据: 1、 摩尔折射率:45.95 2、 摩尔体积(cm3/mol):127.3 3、 等张比容(90.2K):400.7 4、 表面张力(dyne/cm):97.9 5、 极化率(10-24cm3):18.21 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:2 3.氢键受体数量:7 4.可旋转化学键数量:2 5.互变异构体数量:5 6.拓扑分子极性表面积152 7.重原子数量:13 8.表面电荷:0 9.复杂度:297 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:白色针状结晶或结晶性粉末 2. 密度(g/mL,25/4℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):256-261℃(分解) 5. 沸点(ºC,常压):304-305 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):173 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19.溶解性:略溶于沸水,微溶于水,乙醇,几乎不溶于氯仿或乙醚,易溶于氨溶液。 |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Acetazolamide REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 59-66-5 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin irritation (Category 2), H315 Eye irritation (Category 2), H319 For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/EC Xi IrritantR36/38 For the full text of the R-phrases mentioned in this Section, see Section 16. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal wordWarning Hazard statement(s) H315Causes skin irritation. H319Causes serious eye irritation. Precautionary statement(s) P305 + P351 + P338IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Chemical characterization : Natural product Synonyms: N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide Formula: C4H6N4O3S2 Molecular Weight: 222,25 g/mol CAS-No.: 59-66-5 EC-No.: 200-440-5 Hazardous ingredients according to Regulation (EC) No 1272/2008 ComponentClassificationConcentration Acetazolamide CAS-No.59-66-5Skin Irrit. 2; Eye Irrit. 2; H315,<= 100 % EC-No.200-440-5H319 Hazardous ingredients according to Directive 1999/45/EC ComponentClassificationConcentration Acetazolamide CAS-No.59-66-5Xi, R36/38<= 100 % EC-No.200-440-5 For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Sulphur oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Avoid breathing dust. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Light sensitive. Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Full contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) Splash contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an industrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario. Body Protection impervious clothing, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: powder b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingMelting point/range: 258 - 259 °C point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity LD50 Oral - mouse - 4.300 mg/kg Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: AC8225000 Headache, fatigue, Anorexia., Gastrointestinal disturbance, Drowsiness, To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 急性毒性 主要的刺激性影响: 在皮肤上面:可能引起发炎 在眼睛上面:可能引起发炎 致敏作用:没有已知的敏化影响。生态学数据: 总括注解 水危害级别1(德国规例)(通过名单进行自我评估)该物质对水有极其危害的。 不要让未稀释或大量的产品接触地下水、水道或污水系统。 若无政府许可,勿将材料排入周围环境。CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H315-H319 |
| 警示性声明 | P305 + P351 + P338 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xi:Irritant |
| 风险声明 (欧洲) | R36/38 |
| 安全声明 (欧洲) | S26 |
| 危险品运输编码 | 2811 |
| RTECS号 | AC8225000 |
| 包装等级 | III |
| 危险类别 | 6.1 |
| 海关编码 | 2935009090 |
|
~72% 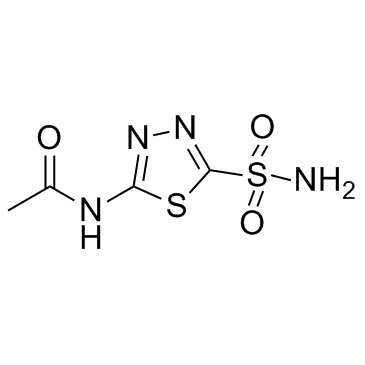
59-66-5 |
| 文献:Tetrahedron Letters, , vol. 54, # 45 p. 5945 - 5947 |
|
~% 
59-66-5 |
| 文献:Journal of the American Chemical Society, , vol. 78, p. 4649,4652 |
|
~% 
59-66-5 |
| 文献:Oriental Journal of Chemistry, , vol. 27, # 1 p. 173 - 178 |
| 上游产品 3 | |
|---|---|
| 下游产品 2 | |
| 海关编码 | 2935009090 |
|---|---|
| 中文概述 | 2935009090 其他磺(酰)胺. 增值税率:17.0% 退税率:9.0% 监管条件:无 最惠国关税:6.5% 普通关税:35.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |