3-Phenylpropan-1-ol
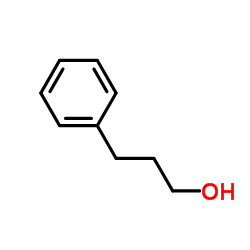
3-Phenylpropan-1-ol structure
|
Common Name | 3-Phenylpropan-1-ol | ||
|---|---|---|---|---|
| CAS Number | 122-97-4 | Molecular Weight | 136.19 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 235.8±9.0 °C at 760 mmHg | |
| Molecular Formula | C9H12O | Melting Point | −18 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 109.4±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Efficient and simple approaches towards direct oxidative esterification of alcohols.
Chemistry 20(47) , 15618-24, (2014) The present article describes novel oxidative protocols for direct esterification of alcohols. The protocols involve successful demonstrations of both "cross" and "self" esterification of a wide variety of alcohols. The cross-esterification proceeds under a s... |
|
|
Intercalation of 3-phenyl-1-proponal into OTS SAMs on silica nanoasperities to create self-repairing interfaces for MEMS lubrication.
Langmuir 26(21) , 16355-61, (2010) Self-assembled monolayers (SAMs) have been widely studied as potential lubricants for microelectromechanical system (MEMS) devices. However, these single-layer films have nominally been found to be insufficient for mitigating wear in sliding contacts because ... |
|
|
Highly efficient, enantioselective syntheses of (S)-(+)- and (R)-(-)-dapoxetine starting with 3-phenyl-1-propanol.
J. Org. Chem. 75(1) , 237-40, (2010) A highly efficient, enantioselective sequence has been developed for the synthesis of (S)- and (R)-dapoxetine. The pathways involve the intermediacy of the 6-membered-ring sulfamate esters 4, which were generated by Du Bois asymmetric C-H amination reactions ... |
|
|
Fragrance material review on 3-phenyl-1-propanol.
Food Chem. Toxicol. 49 Suppl 2 , S246-51, (2011) A toxicologic and dermatologic review of 3-phenyl-1-propanol when used as a fragrance ingredient is presented. 3-Phenyl-1-propanol is a member of the fragrance structural group cinnamyl phenylpropyl compounds. The common characteristic structural element of c... |
|
|
A toxicologic and dermatologic assessment of cinnamyl phenylpropyl materials when used as fragrance ingredients
Food Chem. Toxicol. 49 Suppl 2 , S256-67, (2011) The cinnamyl phenylpropyl fragrance ingredients are a diverse group of chemical structures that have similar metabolic and toxicity profiles. A toxicological and dermatological review of these fragrance ingredients is presented. The common characteristic stru... |
|
|
Improvement in the generation of adsorption isotherm data in the elution by characteristic points method--the ECP-slope approach.
J. Chromatogr. A. 1217(46) , 7215-21, (2010) The elution by characteristic points (ECP) method is a very rapid and precise method for determination of the phase system equilibrium of phase systems in broad solute concentration ranges. Thus, the method is especially suitable for rapid characterization of... |
|
|
Prochiral selectivity in H(2)O(2)-promoted oxidation of arylalkanols catalysed by chloroperoxidase. The role of the interactions between the OH group and the amino-acid residues in the enzyme active site.
Eur. J. Biochem. 268(3) , 665-72, (2001) The H(2)O(2)-promoted oxidations of (R)-[alpha-(2)H(1)]-and (S)-[alpha-(2)H(1)]-arylalkanols catalysed by chloroperoxidase (CPO) from Caldariomyces fumago have been investigated. It has been found that with (R)-[alpha-(2)H(1)]-alcohols, the oxidation involves... |