Methylboronic acid
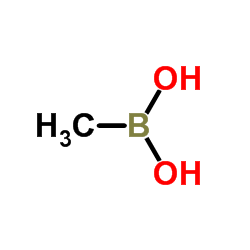
Methylboronic acid structure
|
Common Name | Methylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 13061-96-6 | Molecular Weight | 59.86 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 141.7±23.0 °C at 760 mmHg | |
| Molecular Formula | CH5BO2 | Melting Point | 91-94 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 39.5±22.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Effect of co-ligands on chemical and biological properties of (99m)Tc(III) complexes [(99m)Tc(L)(CDO)(CDOH)2BMe] (L=Cl, F, SCN and N3; CDOH2=cyclohexanedione dioxime).
Nucl. Med. Biol. 41(10) , 813-24, (2014) (99m)Tc-Teboroxime ([(99m)TcCl(CDO)(CDOH)2BMe]) is a member of the BATO (boronic acid adducts of technetium dioximes) class of (99m)Tc(III) complexes. This study sought to explore the impact of co-ligands on solution stability, heart uptake and myocardial ret... |
|
|
Asymmetric bromolactonization using amino-thiocarbamate catalyst.
J. Am. Chem. Soc. 44th ed., 132 , 15474-15476, (2010) A novel amino-thiocarbamate-catalyzed bromolactonization of unsaturated carboxylic acids has been developed. The scope of the reaction is evidenced by 22 examples of γ-lactones with up to 99% yield and 93% ee. The protocol was applied in the enantioselective ... |
|
|
Enantioselective bromoaminocyclization using amino-thiocarbamate catalysts.
J. Am. Chem. Soc. 133 , 9164-9167, (2011) A facile and efficient enantioselective bromoaminocyclization of unsaturated sulfonamides has been developed using an amino-thiocarbamate catalyst. A range of enantioenriched pyrrolidines were prepared with up to 99% yield and 99% ee. The corresponding lactam... |
|
|
Divergent C-H functionalizations directed by sulfonamide pharmacophores: late-stage diversification as a tool for drug discovery.
J. Am. Chem. Soc. 18th ed., 133 , 7222-7228, (2011) Modern drug discovery is contingent on identifying lead compounds and rapidly synthesizing analogues. The use of a common pharmacophore to direct multiple and divergent C-H functionalizations of lead compounds is a particularly attractive approach. Herein, we... |
|
|
Suzuki-Miyaura cross-couplings of secondary allylic boronic esters.
Chem. Commun. (Camb.) 9th ed., 48 , 1230-1232, (2012) Palladium-catalyzed cross-coupling reactions of secondary allylic boronic esters with iodoarenes were demonstrated under the conditions previously described for the coupling of benzylic substrates. The regioselectivity of the process was largely dictated by t... |
|
|
Enol tosylates as viable partners in Pd-catalyzed cross-coupling reactions.
J. Org. Chem. 70 , 10124, (2005) [reaction: see text] Herein we demonstrate functionalized enol tosylates to be robust substrates that undergo Suzuki-Miyaura, Sonogashira, and Stille cross-coupling reactions to provide stereodefined trisubstituted unsaturated esters. |
|
|
A chrysin analog exhibited strong inhibitory activities against both PGE2 and NO production.
Eur. J. Med. Chem. 9th ed., 46 , 4657-4660, (2011) A number of 8-substituted chrysin analogs have been prepared and evaluated for their inhibitory activities of cyclooxygenase-2 catalyzed prostaglandin E(2) and iNOS-mediated NO production. Analogs were prepared via Suzuki-Miyaura C-C cross coupling reaction o... |
|
|
Synlett , 1351, (2006)
|
|
|
Selective heating of Pd-modified ordered mesoporous carbon CMK-3 by microwave irradiation Inagaki, S.; et al.
Bull. Chem. Soc. Jpn. 10th ed., 84 , 1136-1143, (2011)
|
|
|
Ruthenium-catalyzed C-H silylation of methylboronic acid using a removable a-directing modifier on the boron atom Ihara, H.; Ueda, A.; Suginome, M.
Chem. Lett. 9th ed., 40 , 916-918, (2011)
|