bronopol
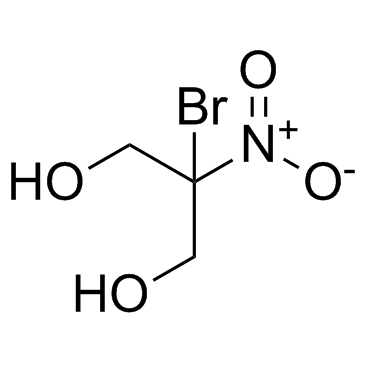
bronopol structure
|
Common Name | bronopol | ||
|---|---|---|---|---|
| CAS Number | 52-51-7 | Molecular Weight | 199.988 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 358.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C3H6BrNO4 | Melting Point | 130-133 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 170.3±27.9 °C | |
| Symbol |



GHS05, GHS07, GHS09 |
Signal Word | Danger | |
|
Non-fragrance allergens in specific cosmetic products.
Contact Dermatitis 65(5) , 276-85, (2011) Reports about the nature of the ingredients responsible for allergic contact dermatitis caused by specific cosmetic products are scarce.Between January 2000 and December 2010, the specific cosmetic products having caused allergic contact dermatitis, as well a... |
|
|
Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives.
J. Am. Acad. Dermatol. 70(1) , 102-7, (2014) Patients with atopic dermatitis (AD) have chronic dry skin to which they frequently apply skin care products containing preservatives, and they are predisposed to developing cutaneous delayed-type hypersensitivity.We sought to compare the rates of positive pa... |
|
|
Patch testing with formaldehyde and formaldehyde-releasers: multicentre study in Spain (2005-2009).
Contact Dermatitis 65(5) , 286-92, (2011) Formaldehyde and formaldehyde-releasers are common causes of allergic contact dermatitis.To determine the frequency of sensitization to formaldehyde and seven formaldehyde-releasers. To establish and characterize groups of patients according to the results of... |
|
|
Patch testing is a useful investigation in children with eczema.
Contact Dermatitis 65(4) , 208-12, (2011) Allergic contact dermatitis in children is less recognized than in adults. However, recently, allergic contact dermatitis has started to attract more interest as a cause of or contributor to eczema in children, and patch testing has been gaining in recognitio... |
|
|
Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 1. Characterization, frequency and relevance of sensitization, and frequency of use in cosmetics.
Contact Dermatitis 62(1) , 2-17, (2010) In this part of a series of review articles on formaldehyde-releasers and their relationship to formaldehyde contact allergy, formaldehyde-releasers in cosmetics are discussed. In this first part of the article, key data are presented including frequency of s... |
|
|
Formaldehyde-releasers in cosmetics in the USA and in Europe.
Contact Dermatitis 62(4) , 221-4, (2010) Frequencies of sensitization to formaldehyde among US patients patch tested for suspected contact dermatitis are higher than in Europe. Cosmetics are an important source of contact with formaldehyde.To acquire data on the frequency of use of formaldehyde-rele... |
|
|
Preparation of active antibacterial LDPE surface through multistep physicochemical approach: I. Allylamine grafting, attachment of antibacterial agent and antibacterial activity assessment.
Colloids Surf. B Biointerfaces 88(1) , 440-7, (2011) Low-density polyethylene (LDPE) samples were treated in air plasma discharge, coated by polyallyamine brush thought copolymeric grafting surface-from reaction and deposited four common antibacterial agents (benzalkonium chloride, bronopol, chlorhexidine and t... |
|
|
Evaluation of the MilkoScan FT 6000 milk analyzer for determining the freezing point of goat's milk under different analytical conditions.
J. Dairy Sci. 90(7) , 3153-61, (2007) The aim of this research was to evaluate the Milko-Scan FT 6000 (Foss Electric, Hillerød, Denmark) for determining the freezing point (FP) of goat's milk under different analytical conditions. The FP was determined in duplicate in 1,800 milk aliquots obtained... |
|
|
Effect of preservatives on the accuracy of mid-infrared milk component testing
J. Dairy Sci. 93(12) , 6000-11, (2010) Our objective was to determine the effect of commonly used milk preservatives on the accuracy of fat, protein, and lactose content determination in milk by mid-infrared (mid-IR) milk analysis. Two producer raw milks (Holstein and Jersey) and 2 pasteurized mod... |
|
|
Toxicity profile of labile preservative bronopol in water: the role of more persistent and toxic transformation products.
Environ. Pollut. 159(2) , 609-15, (2011) Transformation products usually differ in environmental behaviors and toxicological properties from the parent contaminants, and probably cause potential risks to the environment. Toxicity evolution of a labile preservative, bronopol, upon primary aquatic deg... |