4-Methoxyphenylboronic acid
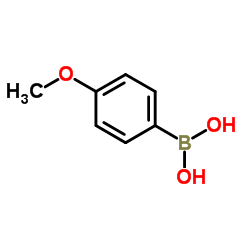
4-Methoxyphenylboronic acid structure
|
Common Name | 4-Methoxyphenylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 5720-07-0 | Molecular Weight | 151.96 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 306.8±44.0 °C at 760 mmHg | |
| Molecular Formula | C7H9BO3 | Melting Point | 204-206 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 139.3±28.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Carbonic anhydrase inhibitors. Inhibition of the fungal beta-carbonic anhydrases from Candida albicans and Cryptococcus neoformans with boronic acids.
Bioorg. Med. Chem. Lett. 19 , 2642-5, (2009) Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalkylboronic acids was investigated. Aromatic, 4-phenylsubstitu... |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combinations of N-(2-pyridyl) substituted benzylamines and arylbo... |
|
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.
Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012) A novel catalytic method for synthesizing 4-aryl-2-quinolinones is reported. The process involves two mechanistically independent, sequential Pd(II)-catalyzed reactions--the oxidative Heck reaction and the intramolecular C-H amidation--both of which smoothly ... |
|
|
An efficient base-free N-arylation of imidazoles and amines with arylboronic acids using copper-exchanged fluorapatite.
J. Org. Chem. 71 , 9522, (2006) N-Arylation of imidazoles and amines with arylboronic acids was accomplished with copper-exchanged fluorapatite (CuFAP) in methanol at room temperature. The products N-arylimidazoles and N-arylamines were isolated in good to excellent yields. A variety of ary... |
|
|
Aryl boronic acid inhibition of synthetic melanin polymerization.
Bioorg. Med. Chem. Lett. 20 , 4475-8, (2010) Inhibitors of melanin formation are sought after for a range of applications. Boronophenylalanine is known to inhibit melanogenesis via boronic acid-catechol interactions. A spectroscopic assay was developed to study the polymerization of l-dopa to synthetic ... |
|
|
Discovery of boronic acids as novel and potent inhibitors of fatty acid amide hydrolase.
J. Med. Chem. 51 , 7057-7060, (2008) A series of commercial phenyl-, heteroaryl-, alkyl-, and alkenylboronic acids were evaluated for their FAAH and MGL inhibitory activities. The compounds were generally selective for FAAH, with IC50 in the nanomolar or low-micromolar range. Eight of these comp... |
|
|
Synthesis and photophysical investigation of a series of push-pull arylvinyldiazine chromophores.
J. Org. Chem. 8th ed., 77 , 4087-4096, (2012) A new series of push-pull arylvinyldiazines has been efficiently prepared by aldol condensation between the appropriate methyldiazine and aromatic aldehyde. The optical absorption and emission properties of these chromophores were studied in different solvent... |
|
|
Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature.
J. Am. Chem. Soc. 15th ed., 134 , 6548-6551, (2012) A Cu-mediated ligandless aerobic fluoroalkylation of arylboronic acids under mild conditions is described for the first time. The reaction tolerates a wide range of functional groups, allowing for further transformation. Mechanistic studies suggest that [R(f)... |
|
|
Rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to nitroalkenes using olefin-sulfoxide ligands.
J. Org. Chem. 7th ed., 77 , 3071-3081, (2012) An efficient rhodium/olefin-sulfoxide catalyzed asymmetric conjugate addition of organoboronic acids to a variety of nitroalkenes has been developed, where 2-methoxy-1-naphthyl sulfinyl functionalized olefin ligands have shown to be highly effective and are a... |
|
|
Pd-catalyzed direct arylation of phenylpyrazole: Synthesis of fipronil derivatives with aryl boronic acids promoted by a stoichiometric amount of NIS Zhang, X-H.; Han, J-S.; Zhong, P.
J. Fluor. Chem. 137 , 44-49, (2012)
|