L-4-Nitrophenylalanine
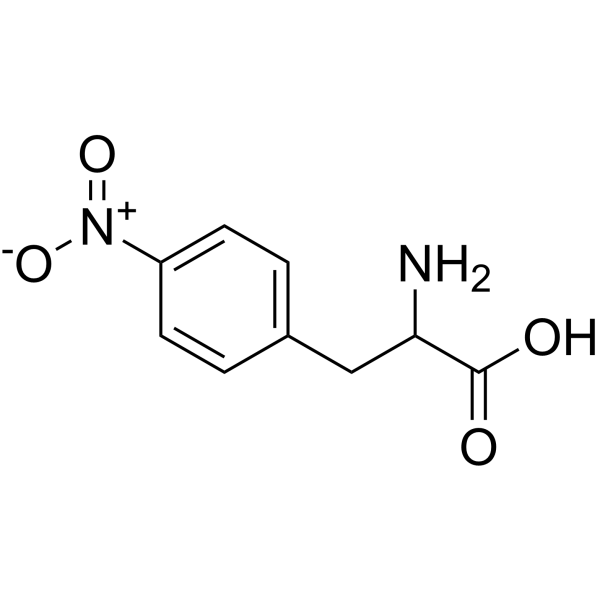
L-4-Nitrophenylalanine structure
|
Common Name | L-4-Nitrophenylalanine | ||
|---|---|---|---|---|
| CAS Number | 2922-40-9 | Molecular Weight | 210.187 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 414.1±35.0 °C at 760 mmHg | |
| Molecular Formula | C9H10N2O4 | Melting Point | 236-237 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 204.2±25.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Sensitive, soluble chromogenic substrates for HIV-1 proteinase.
J. Biol. Chem. 265 , 7733, (1990) By replacement of the P1' residue in a capsid/nucleocapsid cleavage site mimic with 4-NO2-phenylalanine (Nph), an excellent chromogenic substrate, Lys-Ala-Arg-Val-Leu*Nph-Glu-Ala-Met, for HIV-1 proteinase (kappa cat = 20 s-1, Km = 22 microM) has been prepared... |
|
|
Genetic incorporation of unnatural amino acids into proteins in Mycobacterium tuberculosis.
PLoS ONE 5(2) , e9354, (2010) New tools are needed to study the intracellular pathogen Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), to facilitate new drug discovery and vaccine development. We have developed methodology to genetically incorporate unnatural a... |
|
|
Comparison of fluorescence reagents for simultaneous determination of hydroxylated phenylalanine and nitrated tyrosine by high-performance liquid chromatography with fluorescence detection.
Biomed. Chromatogr. 26(1) , 41-50, (2012) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are well-known and important contributors to oxidative and nitrosative stress in several diseases. Hydroxylated phenylalanine and nitrated tyrosine products appear to be particularly susceptibl... |
|
|
A simple chromogenic test for rapid screening of Proteus and Providencia bacteria.
Microbiologica 8(4) , 395-7, (1985) A rapid test for detection of p-nitrophenylalanine ammonia-lyase is described. The test is performed by suspending a loopful of bacteria in 0.5 ml of a buffered 1 mM solution of p-nitro-DL-phenylalanine (PNPA). The enzymatic activity is revealed by the format... |
|
|
Nitro-phenylalanine: a novel sensor for heat transfer in peptides.
J. Phys. Chem. A 115(11) , 2169-75, (2011) Femtosecond IR-pump-IR-probe experiments with independently tunable pulses are used to monitor the ultrafast response of selected IR absorption bands to vibrational excitation of other modes of Fmoc-nitrophenylalanine. The absorptions of both NO(2)-bands chan... |
|
|
Spectroscopically labeled peptaibiotic analogs: the 4-nitrophenylalanine infrared absorption probe inserted at different positions into trichogin GA IV.
J. Pept. Sci. 19(4) , 246-56, (2013) A set of three analogs of the 10-residue, membrane-active lipopeptaibiotic trichogin GA IV, labeled with the promising 4-nitrophenylalanine IR absorption probe for local polarity, was synthesized by the solid-phase methodology, chromatographically purified, a... |
|
|
A continuous fluorometric assay for the feline immunodeficiency virus protease.
Anal. Biochem. 254(2) , 226-30, (1997) A novel fluorogenic substrate for continuous feline immunodeficiency virus (FIV) protease (PR) assay was developed in which 2-aminobenzoic acid (Abz) and p-nitrophenylalanine (F(NO2)) were used as the fluorescent donor and acceptor, respectively. The 14-amino... |
|
|
Fluorogenic peptides containing only alpha-amino acids.
Biochem. Biophys. Res. Commun. 201(2) , 835-40, (1994) Fluorogenic substrates for endopeptidases are often prepared by attaching fluorophores and quenchers to opposite ends of the peptide substrates. Here, we describe a new approach by incorporating tryptophan and p-nitrophenylalanine into peptides to give fluoro... |
|
|
Profiling of proteolytic enzymes in the gut of the tick Ixodes ricinus reveals an evolutionarily conserved network of aspartic and cysteine peptidases.
Parasit. Vectors 1 , 7, (2008) Ticks are vectors for a variety of viral, bacterial and parasitic diseases in human and domestic animals. To survive and reproduce ticks feed on host blood, yet our understanding of the intestinal proteolytic machinery used to derive absorbable nutrients from... |
|
|
Position-specific incorporation of a fluorophore-quencher pair into a single streptavidin through orthogonal four-base codon/anticodon pairs.
J. Am. Chem. Soc. 124(49) , 14586-90, (2002) Four-base codon strategy was applied to incorporate a fluorophore-quencher pair into specific positions on a single protein; beta-anthraniloyl-L-alpha,beta-diaminopropionic acid (atnDap) was employed as a fluorophore and p-nitrophenylalanine (ntrPhe) as a que... |