N-Boc-piperazine
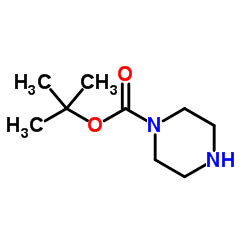
N-Boc-piperazine structure
|
Common Name | N-Boc-piperazine | ||
|---|---|---|---|---|
| CAS Number | 57260-71-6 | Molecular Weight | 186.251 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 258.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H18N2O2 | Melting Point | 47-49ºC | |
| MSDS | Chinese USA | Flash Point | 109.8±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis and dual D2 and 5-HT1A receptor binding affinities of 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones.
J. Enzyme Inhib. Med. Chem. , (2013) A series of new 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones have been synthesized and evaluated for dual D2 and 5-HT1A receptor binding affinities. The synthesized ligands are structurally related to bifeprunox, a potential atypical antipsy... |
|
|
Design, synthesis and structure-activity relationship studies of novel indazole analogues as DNA gyrase inhibitors with Gram-positive antibacterial activity.
Bioorg. Med. Chem. Lett. 14 , 2857-2862, (2004) In this study, we report the design, synthesis and structure-activity relationships of novel indazole derivatives as DNA gyrase inhibitors with Gram-positive antibacterial activity. Our results show that selected compounds from this series exhibit potent anti... |
|
|
Molecular iodine-catalyzed facile procedure for N-Boc protection of amines.
J. Org. Chem. 71 , 8283, (2006) An efficient and practical protocol for the protection of various structurally and electronically divergent aryl and aliphatic amines using (Boc)2O in the presence of a catalytic amount of molecular iodine (10 mol %) under solvent-free conditions at ambient t... |
|
|
Inhibition of noroviruses by piperazine derivatives.
Bioorg. Med. Chem. Lett. 22(1) , 377-9, (2012) There is currently an unmet need for the development of small-molecule therapeutics for norovirus infection. The piperazine scaffold, a privileged structure embodied in many pharmacological agents, was used to synthesize an array of structurally-diverse deriv... |
|
|
1-alkyl-4-acylpiperazines as a new class of imidazole-free histamine H(3) receptor antagonists.
J. Med. Chem. 47 , 2833-2838, (2004) With the aim of identifying structurally novel, centrally acting histamine H(3) antagonists, arrays of monoacyldiamines were screened. This led to the discovery of a series of 1-alkyl-4-acylpiperazines which were potent antagonists at the human histamine H(3)... |
|
|
Efficient copper-catalyzed cross-coupling of 1-Boc-piperazine with aryl iodides and its application in the synthesis of trazodone. Yong FF, et al.
Tetrahedron Lett. 54(39) , 5332-34, (2013)
|
|
|
α,ω-Functionalized Poly (2-Oxazoline) s Bearing Hydroxyl and Amino Functions. Reif M and Jordan R.
Macromol. Chem. Phys. 212(16) , 1815-24, (2011)
|