Gold(1+) chloride-triphenylphosphine (1:1:1)
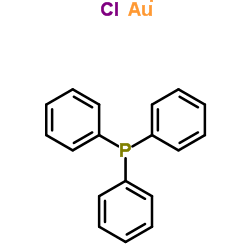
Gold(1+) chloride-triphenylphosphine (1:1:1) structure
|
Common Name | Gold(1+) chloride-triphenylphosphine (1:1:1) | ||
|---|---|---|---|---|
| CAS Number | 14243-64-2 | Molecular Weight | 494.71 | |
| Density | N/A | Boiling Point | 360ºC at 760 mmHg | |
| Molecular Formula | C18H15AuClP | Melting Point | 248-249°C | |
| MSDS | Chinese USA | Flash Point | 181.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A mass spectrometric investigation of the binding of gold antiarthritic agents and the metabolite [Au(CN)2]- to human serum albumin.
J. Biol. Inorg. Chem. 11(5) , 559-70, (2006) Electrospray ionisation (ESI) mass spectrometry was used to examine the reactions of the clinically used antiarthritic agent [Au(S2O3)2]3-, and AuPEt3Cl, a derivative of another clinically used agent auranofin, with human serum albumin (HSA) obtained from a h... |
|
|
Development of a gold-multifaceted catalysis approach to the synthesis of highly substituted pyrroles: mechanistic insights via Huisgen cycloaddition studies.
J. Org. Chem. 78(3) , 920-34, (2013) A novel gold-catalyzed method for the regioselective synthesis of highly substituted pyrroles directly from oximes and alkynes was developed via independent optimization of two key steps of the process. Importantly, a cationic gold(I) species was shown to act... |
|
|
A powerful chiral counterion strategy for asymmetric transition metal catalysis.
Science 317 , 496-499, (2007) Traditionally, transition metal-catalyzed enantioselective transformations rely on chiral ligands tightly bound to the metal to induce asymmetric product distributions. Here we report high enantioselectivities conferred by a chiral counterion in a metal-catal... |
|
|
Au(I)-catalyzed cyclization of enynes bearing an olefinic cycle.
J. Org. Chem. 71 , 9366, (2006) Gold(I)-catalyzed cyclization of enynes containing an olefinic cycle has been studied. The introduction of an olefinic ring instead of a terminal alkene in enynes dramatically increased the yield of the reaction. Enynes having an olefinic cycle were prepared ... |
|
|
Synthesis, structure and cytotoxicity of triphenylphosphinegold(I) sulfanylpropenoates.
J. Inorg. Biochem. 102(2) , 184-92, (2008) The reaction of triphenylphosphinegold(I) chloride in ethanol in a 1:1 molar ratio with the 3-(aryl)-2-sulfanylpropenoic acids H(2)xspa [x: p=3-phenyl-, Clp=3-(2-chlorophenyl)-, -o-mp=3-(2-methoxyphenyl)-, -p-mp=3-(4-methoxyphenyl)-, -o-hp=3-(2-hydroxyphenyl)... |
|
|
Au(PPh(3))Cl-AgSbF(6)-catalyzed rearrangement of propargylic 1,3-dithianes: formation of 8-membered 1,3-bisthio-substituted cyclic allenes.
Chem. Commun. (Camb.) (18) , 2535-7, (2009) Au(PPh(3))Cl-AgSbF(6)-catalyzed rearrangement of propargylic 1,3-dithiane leads to the formation of 8-membered dithio-substituted cyclic allenes, which are remarkably stable. |
|
|
Efficient synthesis of β-enaminones and β-enaminoesters catalyzed by gold (I)/silver (I) under solvent-free conditions.
Molecules 17(3) , 2812-22, (2012) An efficient method for the synthesis of β-enaminones and β-enaminoesters using a combination of [(PPh(3))AuCl]/AgOTf as catalyst has been developed. The reaction between 1,3-dicarbonyl compounds and primary amines was carried out under solvent-free condition... |
|
|
Synlett , 3309, (2006)
|
|
|
Leseurre, L. et al.
Org. Lett. 9 , (2007)
|