5-Chloro-8-hydroxyquinoline
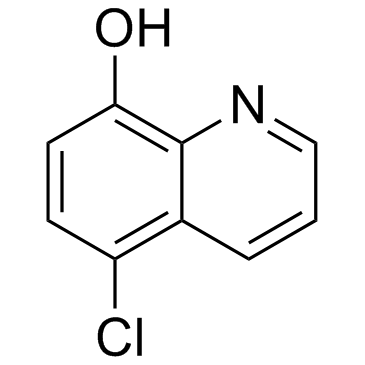
5-Chloro-8-hydroxyquinoline structure
|
Common Name | 5-Chloro-8-hydroxyquinoline | ||
|---|---|---|---|---|
| CAS Number | 130-16-5 | Molecular Weight | 179.603 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 348.7±22.0 °C at 760 mmHg | |
| Molecular Formula | C9H6ClNO | Melting Point | 122-124 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 164.7±22.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Identifying chelators for metalloprotein inhibitors using a fragment-based approach.
J. Med. Chem. 54 , 591-602, (2011) Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced hit rates ranging from 29% to 43% for five matrix metallop... |
|
|
Photoluminescence and thermoanalytical studies of complexes based on 5-Cl-8-hydroxyquinoline and calix[4]arene ligands.
Mater. Sci. Eng. C. Mater. Biol. Appl. 33(4) , 2213-20, (2013) A innovative 5-Cl-8-oxyquinolinepropoxycalix[4]arene ligand (2) have been prepared, exhibiting, at room temperature, blue fluorescent light emission and resulting in shift band to green fluorescent light (fluorescence mode) in the presence of coordinated Eu(I... |
|
|
Poly(L-lactide) and poly(butylene succinate) immiscible blends: from electrospinning to biologically active materials.
Mater. Sci. Eng. C. Mater. Biol. Appl. 41 , 119-26, (2014) For the first time the preparation of defect-free fibers from immiscible blends of high molar mass poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) in the whole range of the polyester weight ratios is shown. Electrospinning using the solvent-nonsolv... |
|
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles from N-oxides.
Org. Lett. 16(3) , 864-7, (2014) A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesi... |
|
|
In vitro activities of cloxyquin (5-chloroquinolin-8-ol) against Mycobacterium tuberculosis.
Antimicrob. Agents Chemother. 51 , 1105-6, (2007) The in vitro activities of cloxyquin (5-chloroquinolin-8-ol) against 9 standard strains and 150 clinical isolates of Mycobacterium tuberculosis were studied. The MICs ranged from 0.062 to 0.25 microg/ml. The MIC(50) and MIC(90) were 0.125 and 0.25 microg/ml, ... |
|
|
Reverse-phase high-performance liquid chromatographic determination of halogenated 8-hydroxyquinoline compounds in pharmaceuticals and bulk drugs.
J. Pharm. Sci. 73(10) , 1430-3, (1984) A reverse-phase high-performance liquid chromatographic (HPLC) method was developed for determining iodochlorhydroxyquin, 5,7-dichloro-8-hydroxyquinoline, and 5,7-diiodo-8-hydroxyquinoline in creams, ointments, shampoos, tablets, and bulk drugs. A column pack... |
|
|
Vibrational spectroscopic investigations, ab initio and DFT studies on 7-bromo-5-chloro-8-hydroxyquinoline.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 72(4) , 783-8, (2009) The Fourier transform infrared (FTIR) and FT-Raman spectra of 7-bromo-5-chloro-8-hydroxyquinoline (BCHQ) have been measured in the range 4000-400 and 4000-100cm(-1), respectively. Complete vibrational assignment and analysis of the fundamental modes of the co... |