Indole-3-pyrubate
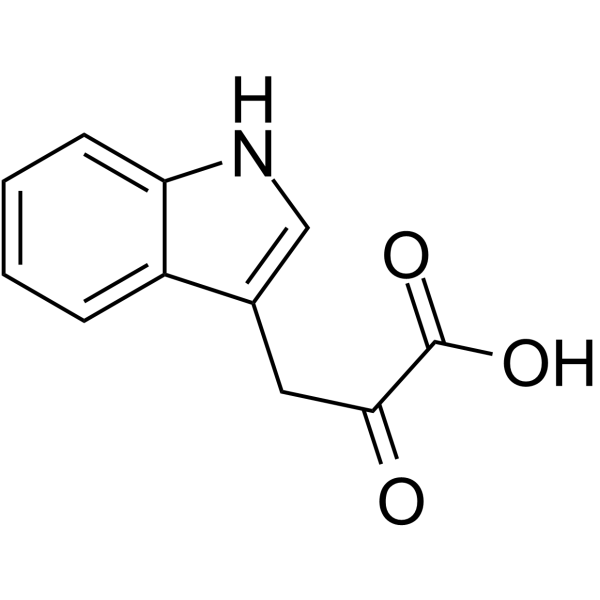
Indole-3-pyrubate structure
|
Common Name | Indole-3-pyrubate | ||
|---|---|---|---|---|
| CAS Number | 392-12-1 | Molecular Weight | 203.194 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 445.2±28.0 °C at 760 mmHg | |
| Molecular Formula | C11H9NO3 | Melting Point | 215 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 223.0±24.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K.
J. Med. Chem. 52 , 2786-93, (2009) Human kynurenine aminotransferase I (hKAT I) catalyzes the formation of kynurenic acid, a neuroactive compound. Here, we report three high-resolution crystal structures (1.50-1.55 A) of hKAT I that are in complex with glycerol and each of two inhibitors of hK... |
|
|
A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis.
Nat. Commun. 6 , 8041, (2015) YUCCA (YUC) proteins constitute a family of flavin monooxygenases (FMOs), with an important role in auxin (IAA) biosynthesis. Here we report that Arabidopsis plants overexpressing YUC6 display enhanced IAA-related phenotypes and exhibit improved drought stres... |
|
|
Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1.
Nat. Chem. Biol. 9(4) , 244-6, (2013) We identify an Arabidopsis pyridoxal-phosphate-dependent aminotransferase, VAS1, whose loss-of-function simultaneously increases amounts of the phytohormone auxin and the ethylene precursor 1-aminocyclopropane-1-carboxylate. VAS1 uses the ethylene biosyntheti... |
|
|
Indole-3-acetic acid biosynthetic pathway and aromatic amino acid aminotransferase activities in Pantoea dispersa strain GPK.
Lett. Appl. Microbiol. 56(5) , 340-7, (2013) This investigation deals with the production of IAA by a bacterial isolate Pantoea dispersa strain GPK (PDG) identified by 16S rRNA gene sequence analysis. HPLC and Mass spectral analysis of metabolites from bacterial spent medium revealed that, IAA productio... |
|
|
High frequency shoots regeneration for mass multiplication of Phyllanthus fraternus Webster--an important antiviral and hepatoprotective plant.
Appl. Biochem. Biotechnol. 169(8) , 2303-14, (2013) An efficient, rapid, and highly reproducible regeneration protocol was successfully developed for Phyllanthus fraternus from the field-derived mature nodal segments. The explants induced multiple shoots on cytokinin containing medium. The highest frequency (9... |
|
|
A new path to auxin.
Nat. Chem. Biol. 4(6) , 337-9, (2008)
|
|
|
A new gene for auxin synthesis.
Cell 133(1) , 31-2, (2008) There is much interest in understanding the pathways that trigger biosynthesis of the plant hormone auxin. In this issue, Stepanova et al. (2008) and Tao et al. (2008) reveal that a small family of tryptophan aminotransferases catalyze formation of indole-3-p... |
|
|
Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers.
Adv. Exp. Med. Biol. 467 , 389-95, (1999) Chemiluminescence associated with oxidation by free radicals was investigated in an alkaline, hemin-catalysed hydrogen peroxide system, using the following tryptophan metabolites as radical scavengers: indole-3-pyruvic, indole-3-propionic, kynurenic, xanthure... |
|
|
D-amino acid oxidase generates agonists of the aryl hydrocarbon receptor from D-tryptophan.
Chem. Res. Toxicol. 22(12) , 1897-904, (2009) The aryl hydrocarbon receptor (AHR) is well-known for its role in mediating the toxic and adaptive responses to xenobiotic compounds. Recent studies also indicate that AHR ligands are endogenously produced and may be essential for normal development. Previous... |
|
|
Functional characterization of the CKRC1/TAA1 gene and dissection of hormonal actions in the Arabidopsis root.
Plant J. 66(3) , 516-27, (2011) Cytokinin (CK) influences many aspects of plant growth and development, and its function often involves intricate interactions with other phytohormones such as auxin and ethylene. However, the molecular mechanisms underlying the role of CK and its interaction... |