tert-Butylamine
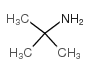
tert-Butylamine structure
|
Common Name | tert-Butylamine | ||
|---|---|---|---|---|
| CAS Number | 75-64-9 | Molecular Weight | 73.13680 | |
| Density | 0.696 g/mL at 25 °C(lit.) | Boiling Point | 46 °C(lit.) | |
| Molecular Formula | C4H11N | Melting Point | -67 °C | |
| MSDS | Chinese USA | Flash Point | −36.4 °F | |
| Symbol |



GHS02, GHS05, GHS06 |
Signal Word | Danger | |
|
Multilayered Thin Films from Boronic Acid-Functional Poly(amido amine)s.
Pharm. Res. 32(9) , 3066-86, (2015) To investigate the properties of phenylboronic acid-functional poly(amido amine) polymers (BA-PAA) in forming multilayered thin films with poly(vinyl alcohol) (PVA) and chondroitin sulfate (ChS), and to evaluate their compatibility with COS-7 cells.Copolymers... |
|
|
Ion chromatography of amylamine and tert.-butylamine in pharmaceuticals.
J. Chromatogr. A. 739(1-2) , 343-9, (1996) Ion chromatographic (IC) methods have been developed for the assay of amylamine in BMS-181 866-02 and tert.-butylamine (TBA) in BMS-188 494-04. BMS-181 866-02 is the penultimate intermediate in the synthesis of a novel thromboxane antagonist, BMS-180 291-02, ... |
|
|
[Hygienic standard for tert-butylamine in water].
Gig. Sanit. (12) , 70-1, (1985)
|
|
|
Quantitative analysis of the mixed activating effects of the alkali metal ions on intestinal brush-border sucrase at pH 5.2.
Biochim. Biophys. Acta 613(1) , 140-52, (1980) The activation of rabbit brush-border sucrase by the alkali metal ions, Li+, Na+ and K+, was analyzed using the equations of the random-order allosteric model previously proposed for sucrase (Mahmood, A. and Alvarado, F. (1975) Arch. Biochem. Biophys. 168, 58... |
|
|
Towards molecular diversity: dealkylation of tert-butyl amine in Ugi-type multicomponent reaction product establishes tert-butyl isocyanide as a useful convertible isonitrile.
Org. Biomol. Chem. 8(16) , 3631-4, (2010) With the development of a novel microwave-assisted one-pot tandem de-tert-butylation of tert-butyl amine in an Ugi-type multicomponent reaction product, tert-butyl isocyanide as a useful convertible isonitrile has been explored for the first time affording ac... |
|
|
Dynamics of an [Fe4S4(SPh)4]2- cluster explored via IR, Raman, and nuclear resonance vibrational spectroscopy (NRVS)-analysis using 36S substitution, DFT calculations, and empirical force fields.
Dalton Trans. (18) , 2192-201, (2006) We have used four vibrational spectroscopies--FT-IR, FT-Raman, resonance Raman, and 57Fe nuclear resonance vibrational spectroscopy (NRVS)--to study the normal modes of the Fe-S cluster in [(n-Bu)4N]2[Fe4S4(SPh)4]. This [Fe4S4(SR)4]2- complex serves as a mode... |
|
|
The Indian drawings of the poet Cesare Pascarella: non-destructive analyses and conservation treatments.
Anal. Bioanal. Chem 402(4) , 1517-28, (2012) The Italian dialect poet Cesare Pascarella travelled all around the world, noting down in notebooks his keen and caustic observations, and drawing sketches that are a visual reportage of his journeys. The sketches were mounted as a random collage over acidic ... |
|
|
Estimation, prevention, and quality control of carbon dioxide loss during aerobic sample processing.
Clin. Chem. 27(10) , 1676-81, (1981) We find that 2 to 6 mmol of carbon dioxide per liter (mean: 4.1 mmol/L) is lost during routine laboratory processing of patients' serum samples after centrifugation. Additional CO2 may be lost if evacuated blood-collection tubes are not filled completely duri... |
|
|
Effects of molecular structures on reduction properties of formyl groups in chlorophylls and pheophytins prepared from oxygenic photosynthetic organisms.
Bioorg. Med. Chem. 19(13) , 3901-5, (2011) Reduction of the 7-formyl groups in chlorophyll (Chl) b and its demetalated compound pheophytin (Phe) b was kinetically analyzed by using tert-butylamine-borane complex (t-BuNH(2)·BH(3)), and was compared with that of the 3-formyl groups in Chl d and Phe d. R... |
|
|
Physico-chemical characterization of a new salt of ibuprofen.
J. Pharm. Biomed. Anal. 8(4) , 321-7, (1990) A new salt of ibuprofen was prepared by reaction with t-butylamine; its formation was confirmed by IR and 1H-NMR spectroscopy. The salt was characterized by thermoanalytical, X-ray powder diffraction and solubility studies. The salt was found to be 1.5 times ... |