Chloroxine
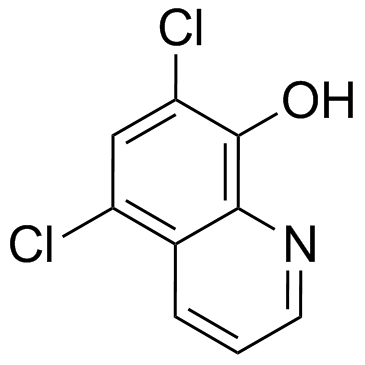
Chloroxine structure
|
Common Name | Chloroxine | ||
|---|---|---|---|---|
| CAS Number | 773-76-2 | Molecular Weight | 214.048 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 354.7±37.0 °C at 760 mmHg | |
| Molecular Formula | C9H5Cl2NO | Melting Point | 178-180 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 168.3±26.5 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles from N-oxides.
Org. Lett. 16(3) , 864-7, (2014) A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesi... |
|
|
Reverse-phase high-performance liquid chromatographic determination of halogenated 8-hydroxyquinoline compounds in pharmaceuticals and bulk drugs.
J. Pharm. Sci. 73(10) , 1430-3, (1984) A reverse-phase high-performance liquid chromatographic (HPLC) method was developed for determining iodochlorhydroxyquin, 5,7-dichloro-8-hydroxyquinoline, and 5,7-diiodo-8-hydroxyquinoline in creams, ointments, shampoos, tablets, and bulk drugs. A column pack... |
|
|
Effects of chlorhexidine, iodine, and 5,7-dichloro-8-hydroxyquinoline on the bacterial composition of rat plaque in vivo.
Caries Res. 18(5) , 440-6, (1984)
|
|
|
[Clinical evaluation on the usefulness of 5,7-dichloro-8-hydroxyquinoline (chloroxine) in association with betamethasone 17-benzoate in the topical treatment of infected cortisone-sensitive dermopathies].
G. Ital. Dermatol. Venereol. 120(2) , XVII-XXIII, (1985)
|
|
|
A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions.
J. Biol. Chem. 279(8) , 6696-700, (2004) Ralstonia eutropha JMP134 2,4,6-trichlorophenol (2,4,6-TCP) 4-monooxygenase catalyzes sequential dechlorinations of 2,4,6-TCP to 6-chlorohydroxyquinol. Although 2,6-dichlorohydroxyquinol is a logical metabolic intermediate, the enzyme hardly uses it as a subs... |
|
|
Fluorimetric determination of chloroxine using manual and flow-injection methods.
J. Pharm. Biomed. Anal. 14(11) , 1505-11, (1996) A reliable and highly sensitive method is described for the determination of chloroxine in pharmaceutical preparations. It involves the formation of a complex between chloroxine and aluminum(III) in a micellar medium. The complex is a very fluorescent species... |
|
|
Genotoxicity of ochratoxin A and structurally related compounds in Escherichia coli strains: studies on their mode of action.
IARC Sci. Publ. (115) , 261-6, (1991) Ochratoxin A, ochratoxin alpha (its major metabolite in rodents) and seven structurally related substances were assayed for SOS DNA repair inducing activity in Escherichia coli PQ37 strain. At a concentration range of 0.1-4 mM, ochratoxin A, chloroxine, 5-chl... |