| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
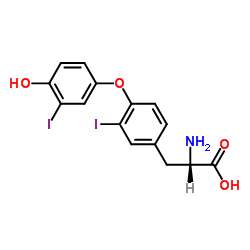 |
3,3'-diiodo-L-thyronine
CAS:4604-41-5 |
|
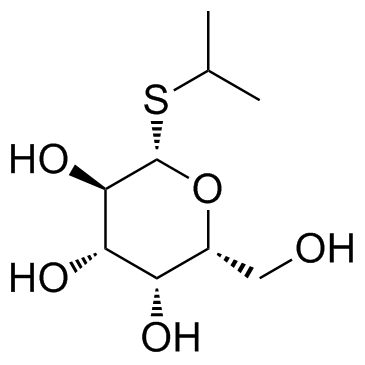 |
Isopropyl-beta-D-thiogalactopyranoside
CAS:367-93-1 |
|
 |
Chloramphenicol
CAS:56-75-7 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
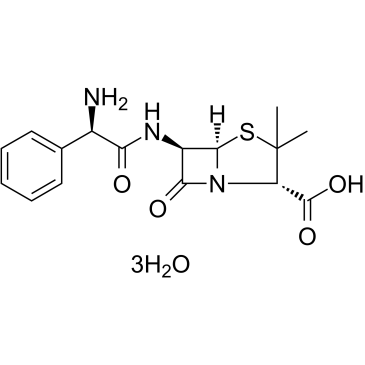 |
Ampicillin Trihydrate
CAS:7177-48-2 |
|
 |
Ampicillin
CAS:69-53-4 |
|
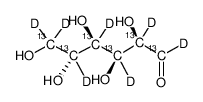 |
D-Glucose-13C6,1,2,3,4,5,6,6-d7
CAS:201417-01-8 |