| Structure | Name/CAS No. | Articles |
|---|---|---|
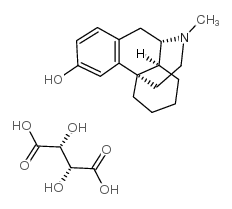 |
DEXTRORPHAN D-TARTRATE
CAS:125-73-5 |
|
 |
Ketoconazole
CAS:65277-42-1 |
|
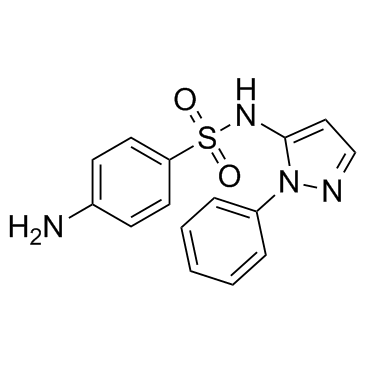 |
Sulfaphenazole
CAS:526-08-9 |
|
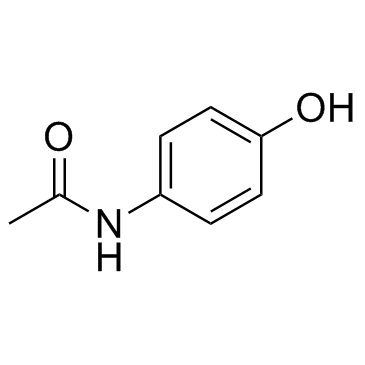 |
4-Acetamidophenol
CAS:103-90-2 |
|
 |
Rosiglitazone
CAS:122320-73-4 |
|
 |
Quinidine
CAS:56-54-2 |
|
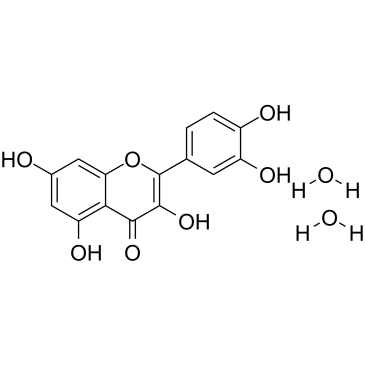 |
Quercetin dihydrate
CAS:6151-25-3 |
|
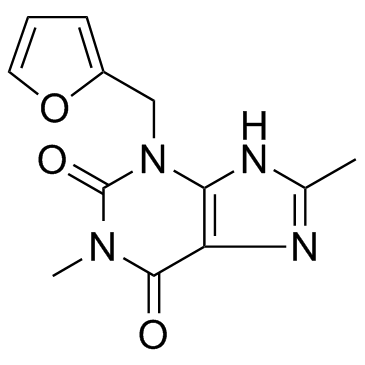 |
Furafylline
CAS:80288-49-9 |
|
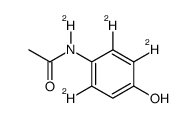 |
Acetaminophen-D4
CAS:64315-36-2 |
|
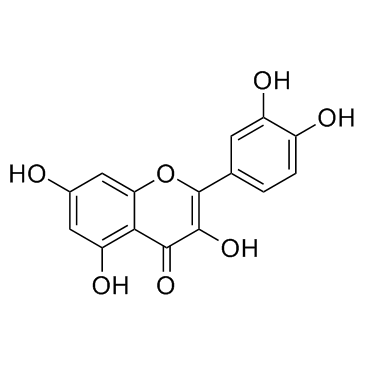 |
Quercetin
CAS:117-39-5 |