| Structure | Name/CAS No. | Articles |
|---|---|---|
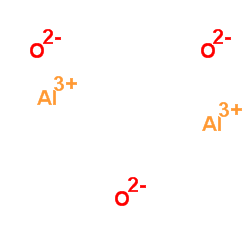 |
Aluminum oxide
CAS:1344-28-1 |
|
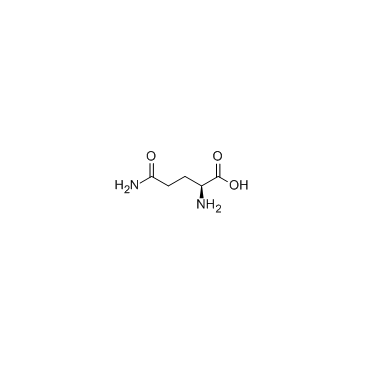 |
L-Glutamine
CAS:56-85-9 |
|
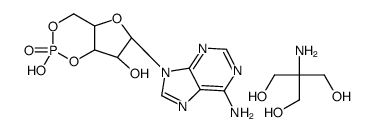 |
ADENOSINE 3':5'-CYCLIC MONOPHOSPHATE TRIS SALT
CAS:102029-77-6 |
|
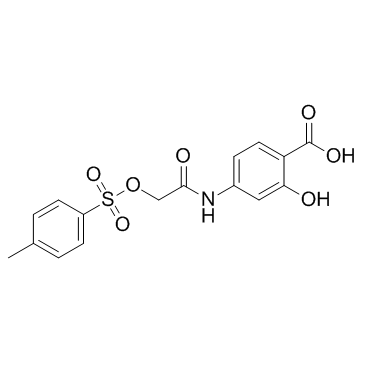 |
S3I-201
CAS:501919-59-1 |
|
 |
Genistein
CAS:446-72-0 |
|
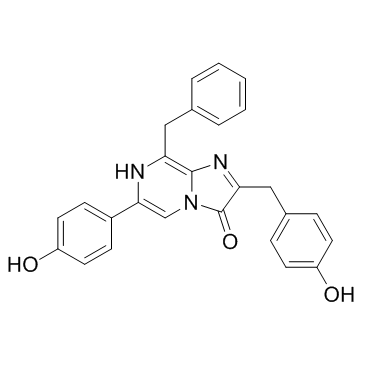 |
Coelenterazine
CAS:55779-48-1 |
|
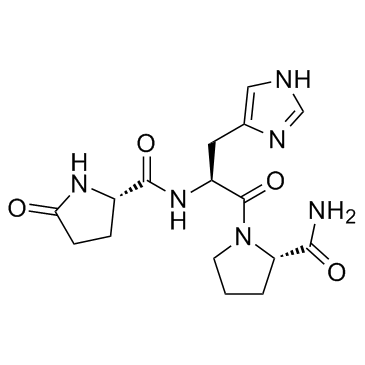 |
TRH
CAS:24305-27-9 |
|
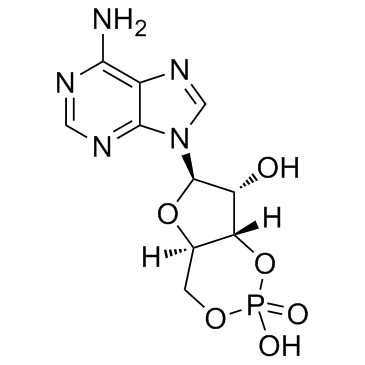 |
Adenosine cyclophosphate
CAS:60-92-4 |