| Structure | Name/CAS No. | Articles |
|---|---|---|
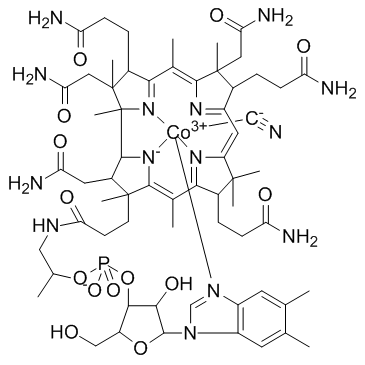 |
Vitamin B12
CAS:68-19-9 |
|
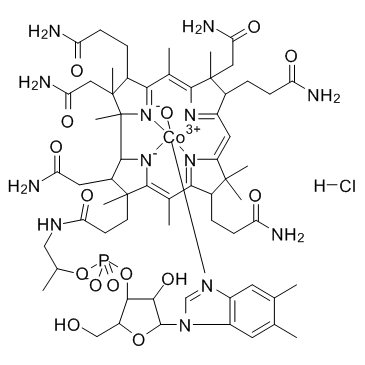 |
Hydroxocobalamin monohydrochloride
CAS:59461-30-2 |
|
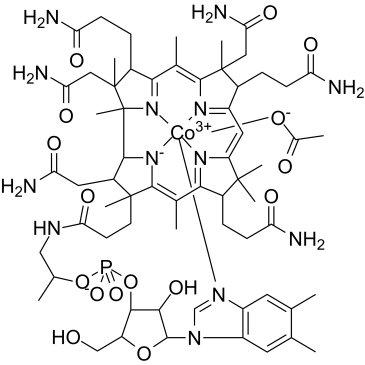 |
Hydroxocobalamin Acetate
CAS:22465-48-1 |
|
 |
Mecobalamin
CAS:13422-55-4 |
|
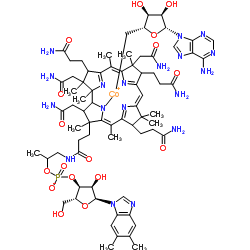 |
coenzyme B12
CAS:13870-90-1 |