N-[(tert-Butoxy)carbonyl]-L-tryptophan
![N-[(tert-Butoxy)carbonyl]-L-tryptophan Structure](https://image.chemsrc.com/caspic/447/13139-14-5.png)
N-[(tert-Butoxy)carbonyl]-L-tryptophan structure
|
Common Name | N-[(tert-Butoxy)carbonyl]-L-tryptophan | ||
|---|---|---|---|---|
| CAS Number | 13139-14-5 | Molecular Weight | 304.341 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 535.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C16H20N2O4 | Melting Point | 136 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 277.8±28.7 °C | |
| Name | N-[(tert-Butoxy)carbonyl]-L-tryptophan |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 535.7±45.0 °C at 760 mmHg |
| Melting Point | 136 °C (dec.)(lit.) |
| Molecular Formula | C16H20N2O4 |
| Molecular Weight | 304.341 |
| Flash Point | 277.8±28.7 °C |
| Exact Mass | 304.142303 |
| PSA | 91.42000 |
| LogP | 2.89 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.602 |
| InChIKey | NFVNYBJCJGKVQK-ZDUSSCGKSA-N |
| SMILES | CC(C)(C)OC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)O |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Oxidative modification of tryptophan residues exposed to peroxynitrite.
Biochem. Biophys. Res. Commun. 234(1) , 82-4, (1997) The aim of this study was to clarify the mechanism of loss of Trp residues in proteins exposed to peroxynitrite. The Trp residues in bovine serum albumin and collagen IV were decreased by peroxynitrit... |
|
|
Abilities of some tryptophan and phenylalanine derivatives to inhibit gastric acid secretion.
Biochim. Biophys. Acta 845(2) , 158-62, (1985) Benzotript (N-p-chlorobenzoyl-L-tryptophan) has been shown to be a receptor-antagonist in vivo and in vitro for peptides from the gastrin family. In the present study, we examine tryptophan, and some ... |
|
|
Methods for determination of electrophoretic mobility and stability of complexes originating in solutions during the chiral discrimination process.
Electrophoresis 19(2) , 276-81, (1998) An equation for the calculation of electrophoretic mobility of kinetically labile complexes originating in solutions during the chiral discrimination process is derived. The mobility of the complex is... |
|
Name: Antifungal activity against Aspergillus flavus after 72 hrs by microdilution method
Source: ChEMBL
Target: Aspergillus flavus
External Id: CHEMBL1670960
|
|
Name: Antifungal activity against Fusarium oxysporum after 72 hrs by microdilution method
Source: ChEMBL
Target: Fusarium oxysporum
External Id: CHEMBL1670961
|
|
Name: qHTS for Inhibitors of Polymerase Kappa
Source: NCGC
Target: DNA polymerase kappa [Homo sapiens]
External Id: PolK100
|
|
Name: Antimicrobial activity against Escherichia coli after 72 hrs by microdilution method
Source: ChEMBL
Target: Escherichia coli
External Id: CHEMBL1670955
|
|
Name: Antimicrobial activity against Xanthomonas oryzae after 72 hrs by microdilution metho...
Source: ChEMBL
Target: Xanthomonas oryzae
External Id: CHEMBL1670956
|
|
Name: Antimicrobial activity against Klebsiella pneumoniae after 72 hrs by microdilution me...
Source: ChEMBL
Target: Klebsiella pneumoniae
External Id: CHEMBL1670957
|
|
Name: Cell-based high throughput primary assay to identify activators of GPR151
Source: The Scripps Research Institute Molecular Screening Center
Target: RecName: Full=G-protein coupled receptor 151; AltName: Full=G-protein coupled receptor PGR7; AltName: Full=GPCR-2037; AltName: Full=Galanin receptor 4; AltName: Full=Galanin-receptor-like protein; Short=GalRL
External Id: GPR151_PHUNTER_AG_LUMI_1536_1X%ACT
|
|
Name: Antimicrobial activity against coagulase positive Staphylococcus after 72 hrs by micr...
Source: ChEMBL
Target: Staphylococcus
External Id: CHEMBL1670958
|
|
Name: Antifungal activity against Aspergillus niger after 72 hrs by microdilution method
Source: ChEMBL
Target: Aspergillus niger
External Id: CHEMBL1670959
|
|
Name: Compound was evaluated for the inhibition of Trypanosoma cruzi LAP (at 30 µM)
Source: ChEMBL
Target: Aminopeptidase
External Id: CHEMBL5305022
|
| N-(tert-Butoxycarbonyl)-L-tryptophan |
| EINECS 236-072-7 |
| Boc-L-Trp |
| (S)-2-((tert-Butoxycarbonyl)amino)-3-(1H-indol-3-yl)propanoic acid |
| MFCD00065595 |
| Boc-D-Trp-OH |
| N-tert-Butoxycarbonyl-L-tryptophan |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-tryptophan |
| Na-(tert-Butoxycarbonyl)-L-tryptophan |
| (2S)-3-(1H-indol-3-yl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid |
| Nalpha-Boc-L-Tryptophane |
| N-Boc-L-tryptophan |
| Boc-Trp-OH |
| Boc-L-tryptophan |
 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:73-22-3
CAS#:73-22-3 CAS#:98015-52-2
CAS#:98015-52-2 CAS#:130384-98-4
CAS#:130384-98-4 CAS#:34619-03-9
CAS#:34619-03-9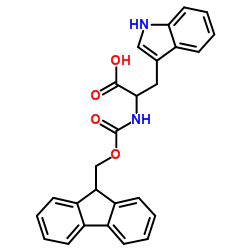 CAS#:35737-15-6
CAS#:35737-15-6 CAS#:64205-15-8
CAS#:64205-15-8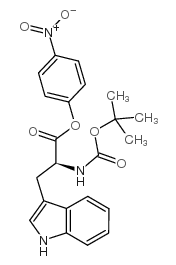 CAS#:15160-31-3
CAS#:15160-31-3 CAS#:62549-92-2
CAS#:62549-92-2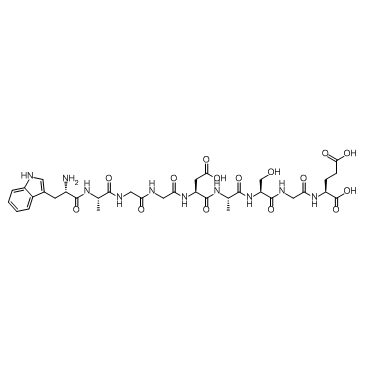 CAS#:62568-57-4
CAS#:62568-57-4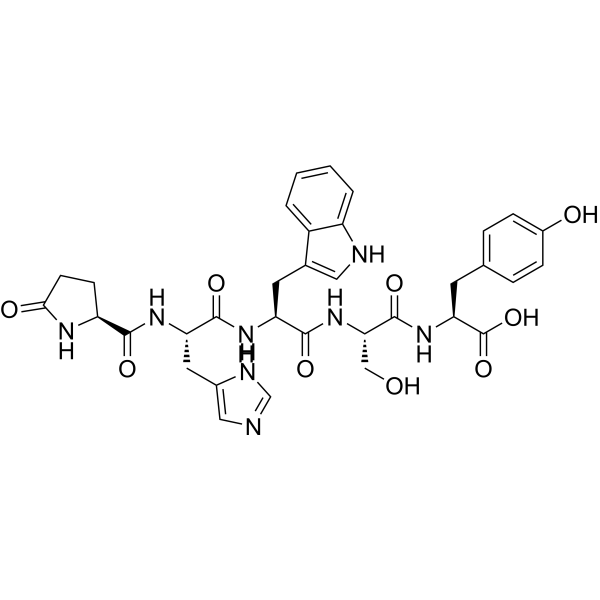 CAS#:52434-75-0
CAS#:52434-75-0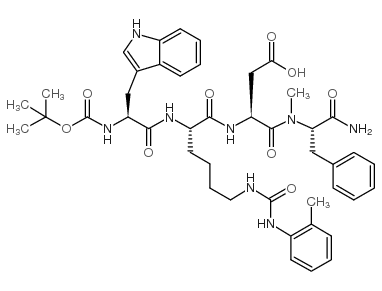 CAS#:130408-77-4
CAS#:130408-77-4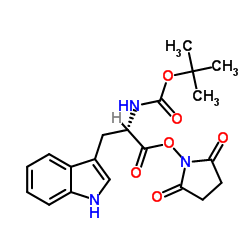 CAS#:3392-11-8
CAS#:3392-11-8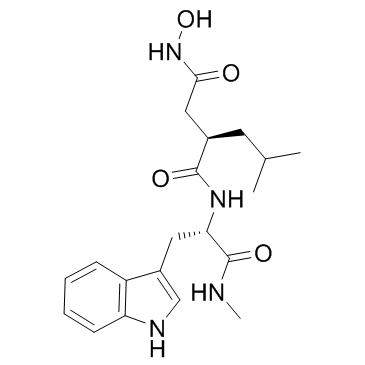 CAS#:142880-36-2
CAS#:142880-36-2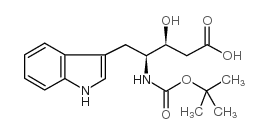 CAS#:109579-23-9
CAS#:109579-23-9 CAS#:229639-48-9
CAS#:229639-48-9
