1H-Indole-3-carboxylic acid
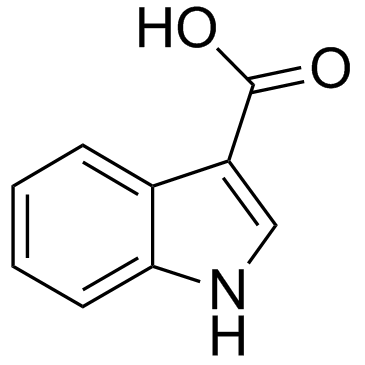
1H-Indole-3-carboxylic acid structure
|
Common Name | 1H-Indole-3-carboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 771-50-6 | Molecular Weight | 161.157 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 419.6±18.0 °C at 760 mmHg | |
| Molecular Formula | C9H7NO2 | Melting Point | 232-234 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 207.6±21.2 °C | |
Use of 1H-Indole-3-carboxylic acidIndole-3-carboxylic acid is a normal urinary indolic tryptophan metabolite and has been found elevated in patients with liver diseases[1][2]. |
| Name | indole-3-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Indole-3-carboxylic acid is a normal urinary indolic tryptophan metabolite and has been found elevated in patients with liver diseases[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 419.6±18.0 °C at 760 mmHg |
| Melting Point | 232-234 °C (dec.)(lit.) |
| Molecular Formula | C9H7NO2 |
| Molecular Weight | 161.157 |
| Flash Point | 207.6±21.2 °C |
| Exact Mass | 161.047684 |
| PSA | 53.09000 |
| LogP | 1.99 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.726 |
| InChIKey | KMAKOBLIOCQGJP-UHFFFAOYSA-N |
| SMILES | O=C(O)c1c[nH]c2ccccc12 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R21/22;R36/37/38 |
| Safety Phrases | S22-S24/25-S36/37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NK7882892 |
| HS Code | 2942000000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
QSAR study on permeability of hydrophobic compounds with artificial membranes.
Bioorg. Med. Chem. 15 , 3756-67, (2007) We previously reported a classical quantitative structure-activity relationship (QSAR) equation for permeability coefficients (P(app-pampa)) by parallel artificial membrane permeation assay (PAMPA) of... |
|
|
Isolation and characterization of products from the nitrosation of the alkaloid gramine.
Food Chem. Toxicol. 23(9) , 841-7, (1985) The nitrosation of gramine, a tertiary amine alkaloid present in barley malt, was carried out by reaction with sodium nitrite in buffered acetic acid (pH 3.4) for 1 hr at room temperature. Two major n... |
|
|
Total synthesis of kottamide E.
Chem. Commun. (Camb.) 49(23) , 2296-8, (2013) The first synthesis of kottamide E, a marine natural product containing a 5,6-dibromoindole linked via a (Z)-enamide to an unusual 1,2-dithiolane-containing amino acid, is reported. |
|
Name: Inverse agonist activity at Gal4-fused human Nurr1 LBD expressed in HEK293T cells co-...
Source: ChEMBL
Target: Nuclear receptor subfamily 4 group A member 2
External Id: CHEMBL4840093
|
|
Name: USP8 deubiquitinase inhibition: Primary qHTS
Source: 24642
Target: ubiquitin specific peptidase 8
External Id: USP8 FAST DUB HTS Primary
|
|
Name: Antioxidant activity assessed as trolox equivalents of ABTS radical scavenging activi...
Source: ChEMBL
Target: NON-PROTEIN TARGET
External Id: CHEMBL990240
|
|
Name: Partition coefficient, log P of the compound
Source: ChEMBL
Target: N/A
External Id: CHEMBL905620
|
|
Name: Experimentally measured binding affinity data (Kd) for protein-ligand complexes deriv...
Source: Shanghai Institute of Organic Chemistry
Target: N/A
External Id: PDBbind-Kd for protein-ligand complexes
|
|
Name: USP17 deubiquitinase inhibition: Primary qHTS
Source: 24642
Target: ubiquitin specific peptidase 17 like family member 5
External Id: USP17 FAST DUB HTS Primary
|
|
Name: Dissociation constant, pKa of the compound by dip probe absorption spectroscopy
Source: ChEMBL
Target: N/A
External Id: CHEMBL5122665
|
|
Name: USP7 deubiquitinase inhibition: Primary qHTS
Source: 24642
Target: ubiquitin specific peptidase 7
External Id: USP7 FAST DUB HTS Primary
|
|
Name: Antioxidant activity against beta-carotene and linoleic acid assessed asbleaching of ...
Source: ChEMBL
Target: NON-PROTEIN TARGET
External Id: CHEMBL990241
|
|
Name: Antibacterial activity against Staphylococcus aureus MRSA ATCC 43300 (CO-ADD:GP_020);...
Source: ChEMBL
Target: Staphylococcus aureus
External Id: CHEMBL4296184
|
| 3-Indolylcarboxylic acid |
| EINECS 212-231-6 |
| Indole-3-carboxylic acid |
| Indole-3-carboxylicacid |
| 1H-Indole-3-carboxylic acid |
| MFCD00005624 |
| Indole-3-carboxylic_acid |
| 3-Indoleformic acid |
| 3-Indolecarboxylic acid |
 CAS#:120-72-9
CAS#:120-72-9 CAS#:124-38-9
CAS#:124-38-9 CAS#:5457-28-3
CAS#:5457-28-3 CAS#:83451-61-0
CAS#:83451-61-0 CAS#:14618-45-2
CAS#:14618-45-2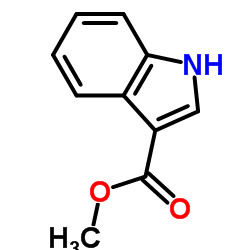 CAS#:942-24-5
CAS#:942-24-5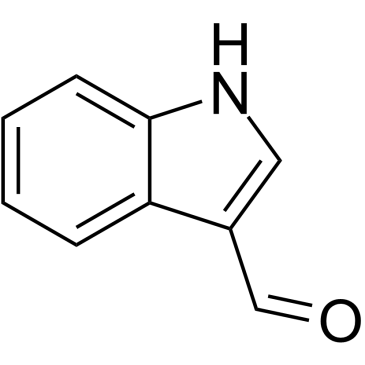 CAS#:487-89-8
CAS#:487-89-8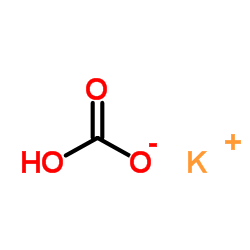 CAS#:298-14-6
CAS#:298-14-6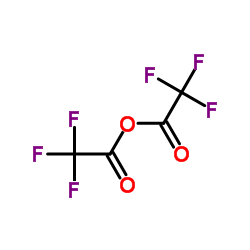 CAS#:407-25-0
CAS#:407-25-0 CAS#:2592-05-4
CAS#:2592-05-4 CAS#:32387-21-6
CAS#:32387-21-6 CAS#:108438-43-3
CAS#:108438-43-3 CAS#:39891-70-8
CAS#:39891-70-8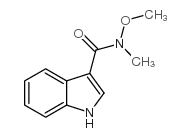 CAS#:214759-95-2
CAS#:214759-95-2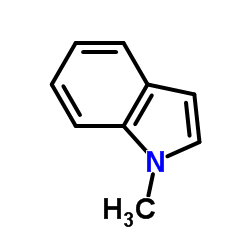 CAS#:603-76-9
CAS#:603-76-9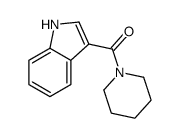 CAS#:27393-79-9
CAS#:27393-79-9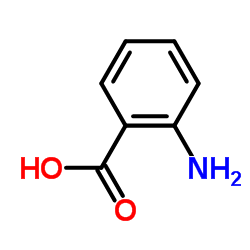 CAS#:118-92-3
CAS#:118-92-3 CAS#:482-89-3
CAS#:482-89-3