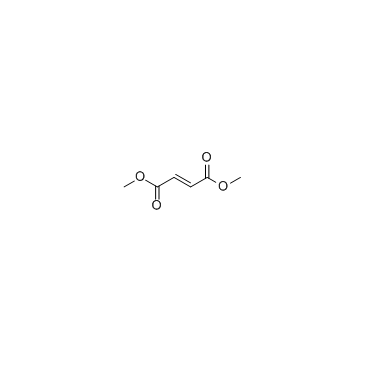
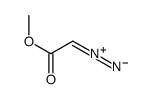
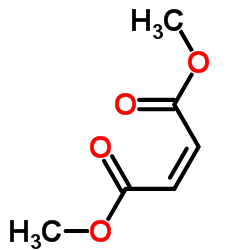
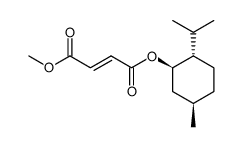
624-49-7
| Name | dimethyl fumarate |
|---|---|
| Synonyms |
Dimethyl fumarate
EINECS 210-849-0 MFCD00064438 |
| Description | Dimethyl fumarate is a nuclear factor (erythroid-derived)-like 2 (Nrf2) pathway activator and induces upregulation of antioxidant gene expression. |
|---|---|
| Related Catalog | |
| In Vitro | Dimethyl fumarate causes short-lived oxidative stress, which leads to increased levels and nuclear localization of the transcription factor nuclear factor erythroid 2-related factor 2 and a subsequent increase in glutathione synthesis and recycling in neuronal cells[1]. Dimethyl fumarate inhibits dendritic cell (DC) maturation by reducing inflammatory cytokine production (IL-12 and IL-6) and the expression of MHC class II, CD80, and CD86. Dimethyl fumarate impairs nuclear factor κB (NF-κB) signaling via reduced p65 nuclear translocalization and phosphorylation. Dimethyl fumarate inhibits maturation of DCs and subsequently Th1 and Th17 cell differentiation by suppression of both NF-κB and ERK1/2-MSK1 signaling[2]. Dimethyl fumarate inhibits TNF-alpha-induced nuclear entry of NF-kappaB in rat heart endothelial cells (RHEC)[3]. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and neurotoxin release. Dimethyl fumarate attenuates CCL2-induced monocyte chemotaxis, suggesting that Dimethyl fumarate could decrease recruitment of activated monocytes to the CNS in response to inflammatory mediators[4]. |
| In Vivo | Dimethyl fumarate inhibits nuclear entry of NF-kappaB in RHEC and reduces myocardial infarct size after ischemia and reperfusion in rats in vivo[3]. Dimethyl fumarate oral administration is shown to upregulate mRNA and protein levels of Nrf2 and Nrf2-regulated cytoprotective genes, attenuate 6-OHDA induced striatal oxidative stress and inflammation in C57BL/6 mice[5]. |
| Animal Admin | In a blinded design, the rats are assigned to one of the three experimental groups: a) to the DMF group receiving 10 mg/kg body weight DMF (dissolved in DMSO 2% in water), or b) to the vehicle group receiving only the vehicle (DMSO 2% in water; necessary to dissolve DMF), or c) to the positive control group receiving the vehicle plus ischemic preconditioning (IPC, known to reduce infarct size). This dose of 10 mg/kg body weight of DMF corresponded approximately to a maximally tolerated dose in man. As we could confirm in our in vitro experiments that DMF is the active compound, the in vivo experiments are performed with DMF but not with MHF. DMF and the vehicle, respectively, tail vein is administrated by i.v. 90 min before ischemia (under general anesthesia using isoflurane) as well as immediately before ischemia. Ischemic preconditioning is induced by two times 5 min episodes ischemia (induced by left coronary artery occlusion) each followed by 5 min of reperfusion (induced by releasing the snare). The experiments are performed (except pilot experiments) pair wise, i.e. two experiments in parallel avoiding identical group assignment. The present study are set up, and permitted to demonstrate a pharmacodynamic effect of DMF on myocardial infarct size in rats in vivo. An eventual mode of action should be investigated in vitro. The results from our in vitro experiments as well as results of other laboratories did not show any effect of MHF on NF-κB activation. Therefore, no experiment is included to exclude MHF from the mode of action of DMF. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 193.0±0.0 °C at 760 mmHg |
| Melting Point | 102-106 °C(lit.) |
| Molecular Formula | C6H8O4 |
| Molecular Weight | 144.125 |
| Flash Point | 91.1±0.0 °C |
| Exact Mass | 144.042252 |
| PSA | 52.60000 |
| LogP | 0.62 |
| Vapour Pressure | 0.5±0.3 mmHg at 25°C |
| Index of Refraction | 1.435 |
| Storage condition | Store at RT. |
| Stability | Stable. Incompatible with acids, bases, oxidizing agents, reducing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H312-H315-H317-H319-H411 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R21;R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 1 |
| RTECS | EM6125000 |
| HS Code | 2917140000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2917190090 |
|---|---|
| Summary | 2917190090 acyclic polycarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |