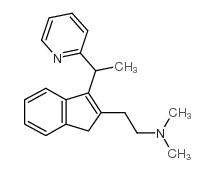
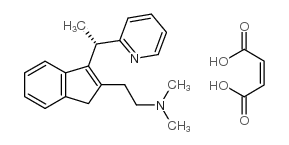
5636-83-9
| Name | N,N-dimethyl-2-[3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethanamine |
|---|---|
| Synonyms |
Triten
Dimethpyrindene Dimetindeno N,N-dimethyl-3-[1-(2-pyridyl)ethyl]inden-2-ethylamine Dimetindene dimethyl-{2-[3-(1-pyridin-2-yl-ethyl)-inden-2-yl]-ethyl}-amine Pecofenil dimethindene Dimetindenum Foristal Fenistil Forhistal |
| Description | Dimethindene is a potent, selective histamine H1 antagonist. Dimethindene impairs cutaneous wound healing (WH). Dimethindene can block K+ currents[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Dimethindene (5-500 μM; follicle-enclosed Xenopus oocytes) decreases Cromakalim cromakalim-induced K+ currents with an IC50 value of 29.5 μM[2]. |
| In Vivo | Dimethindene (0.25 mg; i.p.; once; C57BL/6 mice with skin WH) impaires cutaneous wound healing (WH) and delays skin wound closure[1]. Animal Model: C57BL/6 mice with skin WH[1] Dosage: 0.25 mg Administration: intraperitoneal injection; once Result: Delayed skin wound closure as compared to vehicle treated mice. |
| Density | 1.065 g/cm3 |
|---|---|
| Boiling Point | 416.3ºC at 760 mmHg |
| Melting Point | 50 - 53 °C |
| Molecular Formula | C20H24N2 |
| Molecular Weight | 292.41800 |
| Flash Point | 205.6ºC |
| Exact Mass | 292.19400 |
| PSA | 16.13000 |
| LogP | 4.14670 |
| Index of Refraction | 1.587 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 0 | |
|---|---|
| DownStream 1 | |