34391-04-3
| Name | (R)-salbutamol |
|---|---|
| Synonyms |
(-)-Albuterol
(R)-albuterol R-Albuterol Levosalbutamol (a1R)-a1-[[(1,1-Dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol (l)-albuterol (R)-a1-((tert-Butylamino)methyl)-4-hydroxy-m-xylene-a,a'-diol (-)-a1-(((1,1-Dimethylethyl)amino)methyl)-4-hydroxy-1,3-benzenedimethanol (R)-salbutamol 4-[(1R)-2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol 2-(Hydroxymethyl)-4-{(1R)-1-hydroxy-2-[(2-methyl-2-propanyl)amino]ethyl}phenol R-Salbutamol UNII-EDN2NBH5SS (-)-Salbutamol Levalbuterol Albuterol MFCD00869868 Salbutamol |
| Description | Levalbuterol ((R)-Albuterol; (R)-Salbutamol) is a short-acting β2-adrenergic receptor agonist and the active (R)-enantiomer of Salbutamol. Levalbuterol is a more potent bronchodilator than Salbutamol and has the potential for the treatment of COPD[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Levalbuterol (10 μM; 24 hours) induces 11β-HSD1 mRNA expression, however, it does not influence 11β-HSD2expression in airway epithelial cells[1]. Levalbuterol (10 μM; 24 hours) significantly reduces both LPS- and TNF-α-induced NF-κB activity while increasing GRE activation in an 11β-HSD1 dependent manner in a transformed mouse airway epithelial cell line[1]. RT-PCR[1] Cell Line: Murine Club (MTCC) cells Concentration: 10 μM Incubation Time: 24 hours Result: Increased 11β-HSD1 mRNA expression selectively. |
| In Vivo | Levalbuterol (subcutaneous injection; 1 mg/kg; 14 days) significantly decreases pulmonary inflammation in OVA mice, demonstrated a decrease in eosinophilia and IgE[3]. Animal Model: C57BL/6 female mice with a pulmonary allergic model[3] Dosage: 1 mg/kg Administration: Subcutaneous injection; 1 mg/kg; 14 days Result: Decreased pulmonary inflammation after OVA sensitization. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 433.5±40.0 °C at 760 mmHg |
| Molecular Formula | C13H21NO3 |
| Molecular Weight | 239.311 |
| Flash Point | 159.5±17.9 °C |
| Exact Mass | 239.152145 |
| PSA | 72.72000 |
| LogP | 0.01 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.566 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| HS Code | 2922509090 |
|
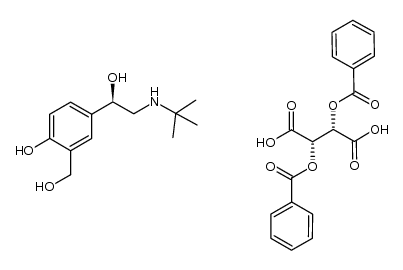
~93% 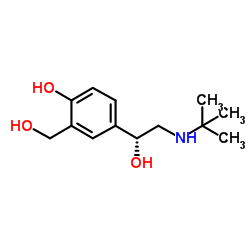
34391-04-3 |
| Literature: Merli, Valeriano; Mantovani, Silvia; Bianchi, Stefano; Daverio, Paola Patent: US2005/261368 A1, 2005 ; Location in patent: Page/Page column 7 ; |
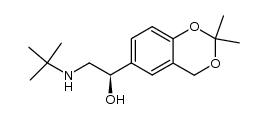
|
~95%
Detail
|
| Literature: Merli, Valeriano; Mantovani, Silvia; Bianchi, Stefano; Daverio, Paola Patent: US2005/261368 A1, 2005 ; Location in patent: Page/Page column 8-10 ; |
|
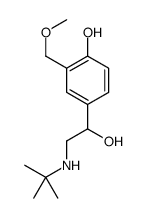
~10% 
34391-04-3 |
| Literature: Fine Chemical Corporation Limited Patent: US6365756 B1, 2002 ; Location in patent: Example 13 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |